Product Information
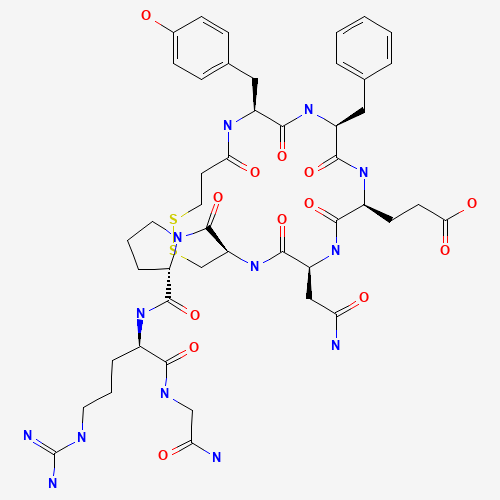
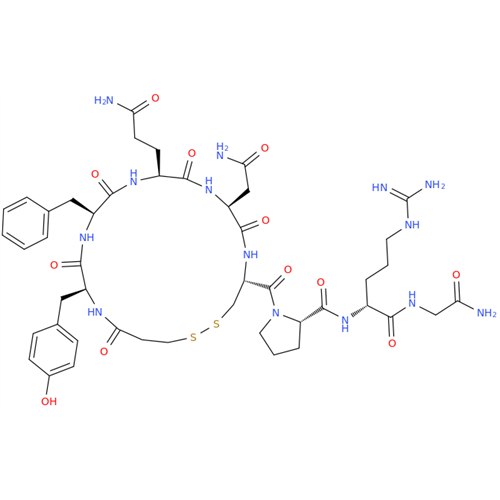
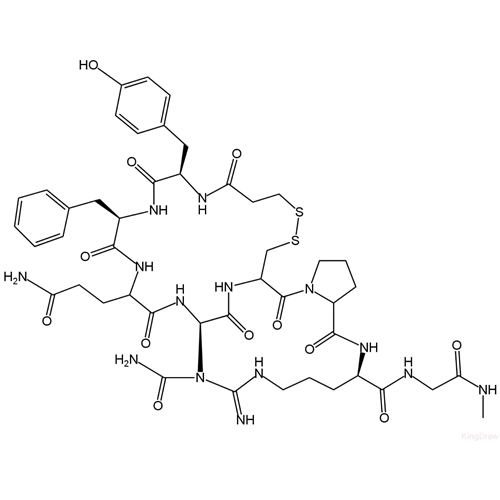
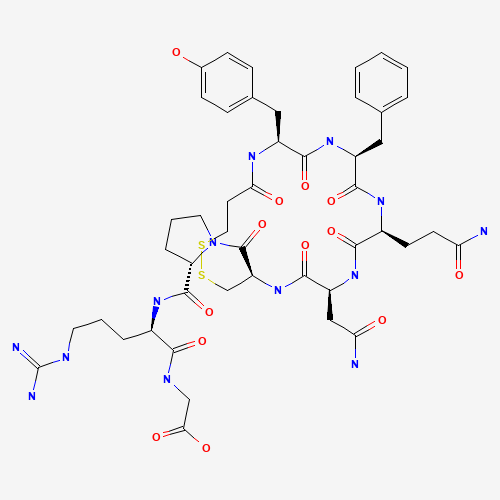
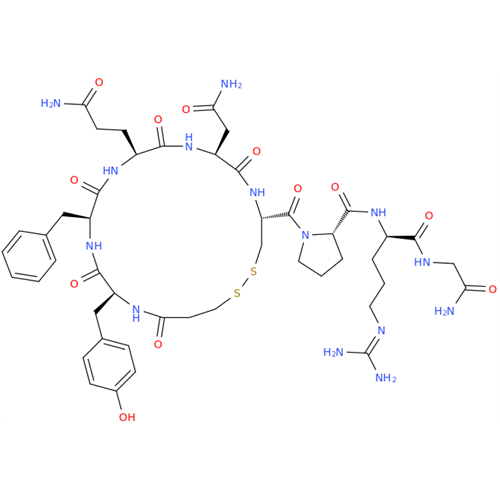
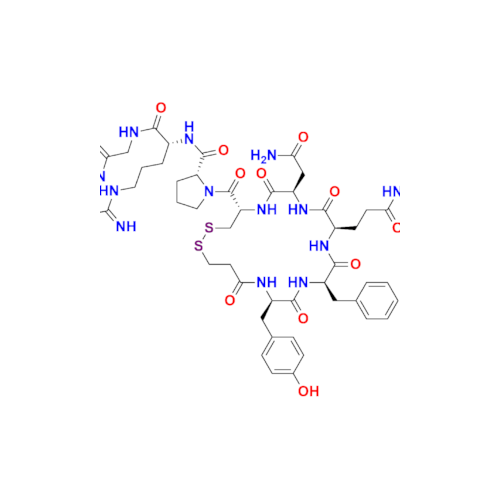
Desmopressin EP Impurity E
|
Chemical Name: Desmopressin EP Impurity E
Synonym: [Gln4(Acm)] desmopressin| Enter Batch Number | |||