Product Information
Clobetasol Propionate EP Impurity J
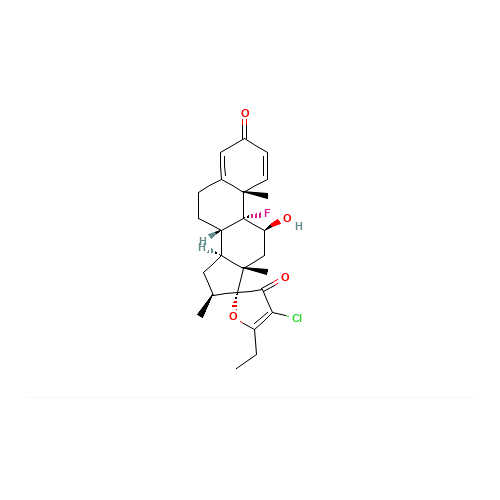
|
Chemical Name: Clobetasol Propionate EP Impurity J
Synonym: Clobetasol Propionate USP Related Compound A ; 17α-spiro compound| Enter Batch Number | |||


