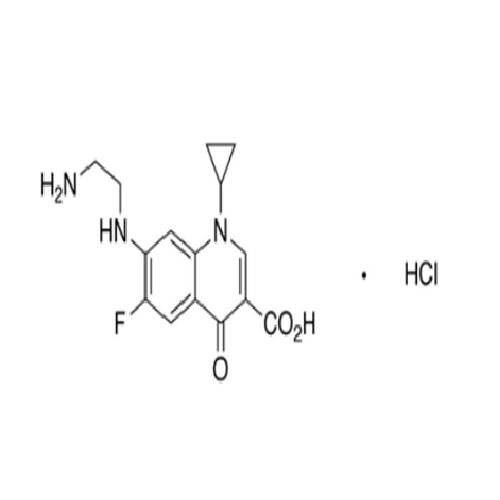
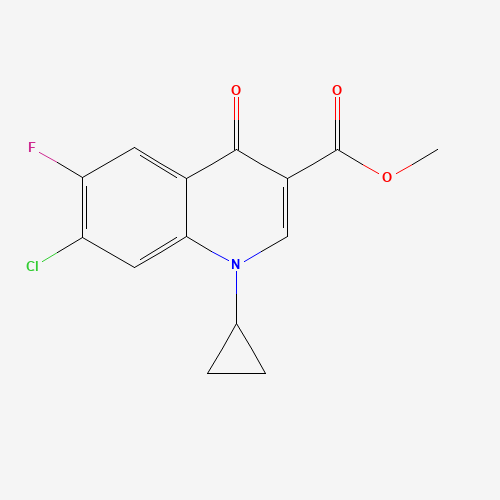
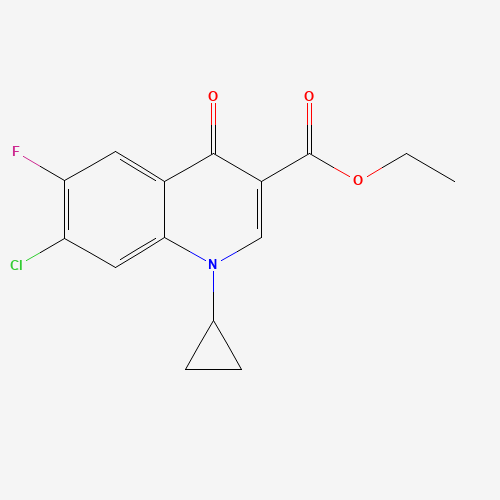
Product Information
Ciprofloxacin Impurity 12
|
Chemical Name: Ciprofloxacin Impurity 12
Synonym: Ethyl 7-chloro-1-cyclopropyl-6-fluoro-4-oxoquinoline-3-carboxylate; Ethyl 7-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-3-quinolinecarboxylate| Enter Batch Number | |||