Product Information
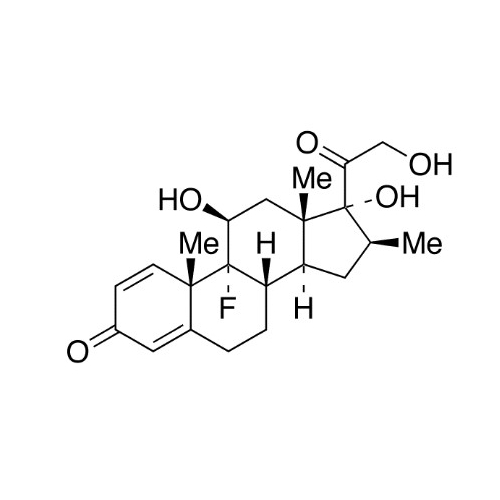
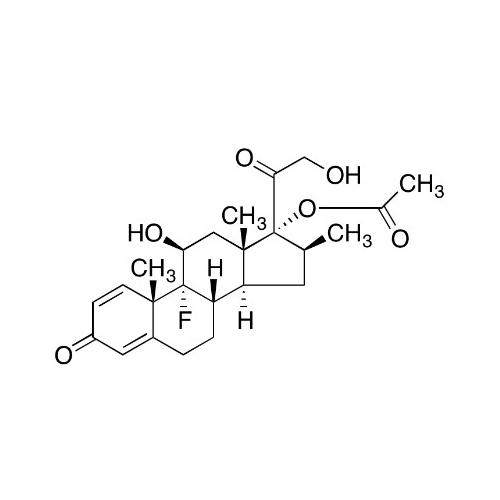
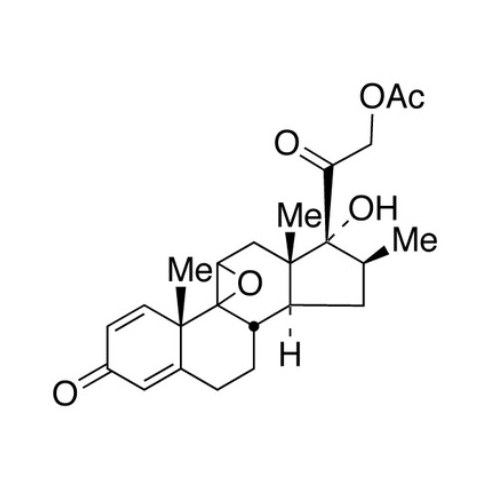
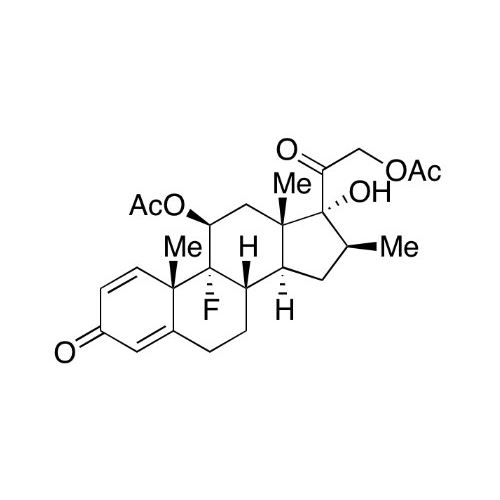
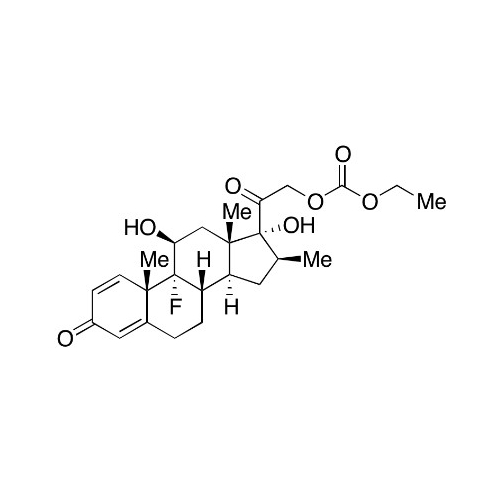
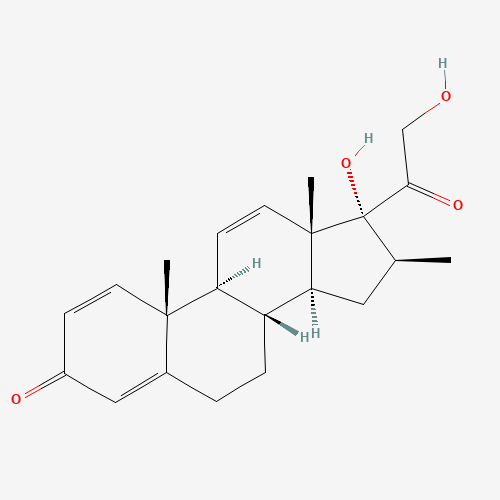
Betamethasone EP Impurity F
|
Chemical Name: Betamethasone EP Impurity F
Synonym: 17,21-Dihydroxy-16beta-methylpregna-1,4,11-triene-3,20-dione| Enter Batch Number | |||