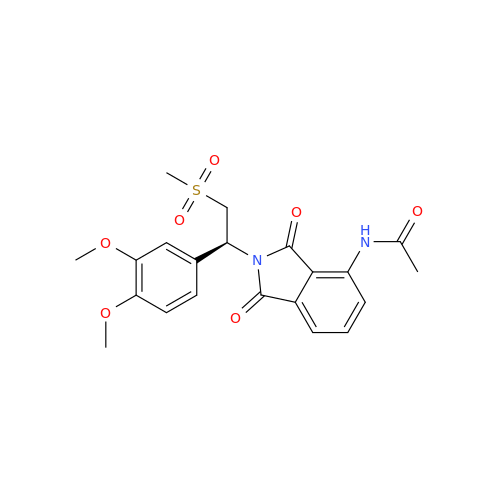
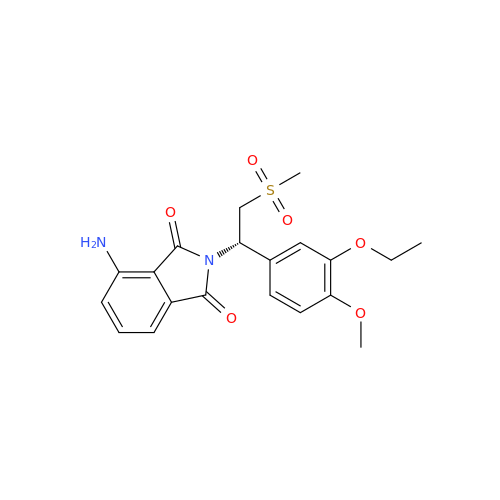
Product Information
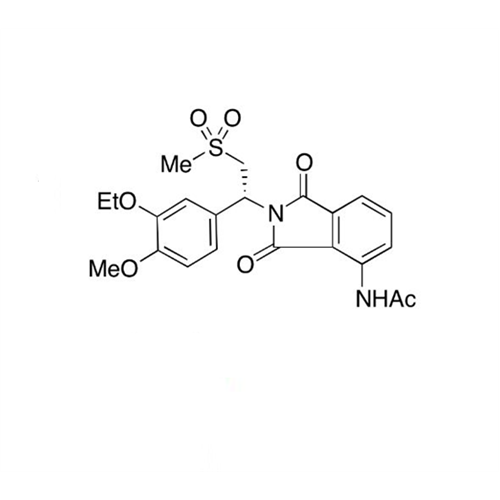
Apremilast EP Impurity H
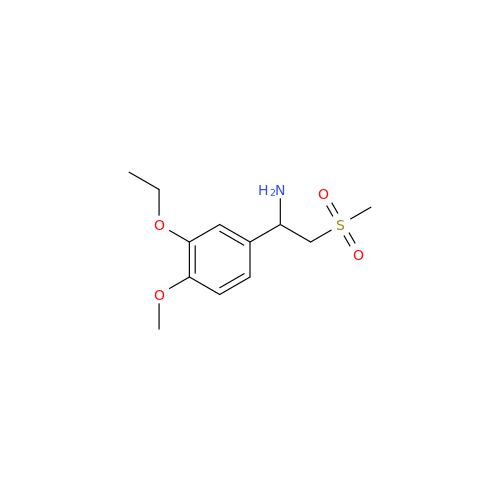
|
Chemical Name: Apremilast EP Impurity H
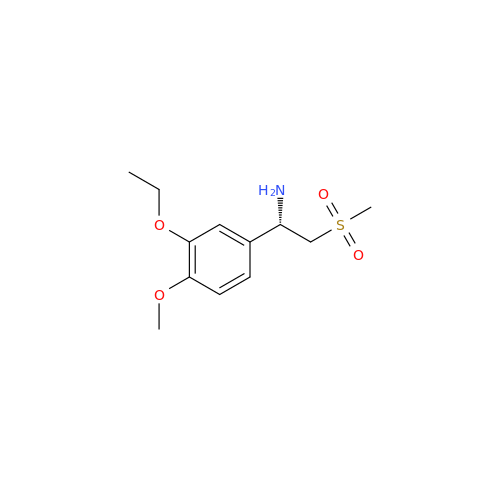
Synonym: 3-Ethoxy-4-methoxy-α-[(methylsulfonyl)methyl]benzenemethanamine; 2-(3-Ethoxy-4-methoxyphenyl)-1-(methylsulfonyl)eth-2-ylamine; Apremilast amine; 1-(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethan-1-amine (As per USP Commenting Opening 51(2))| Enter Batch Number | |||