Product Information
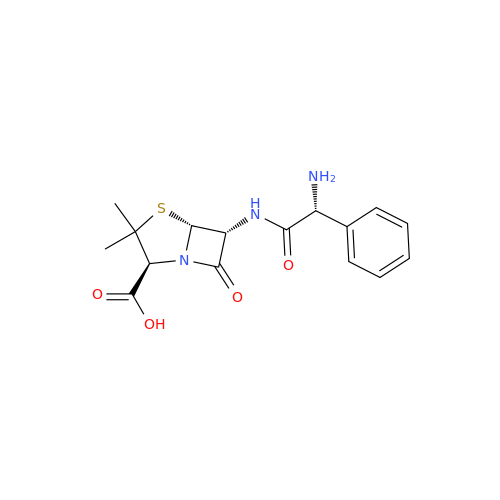
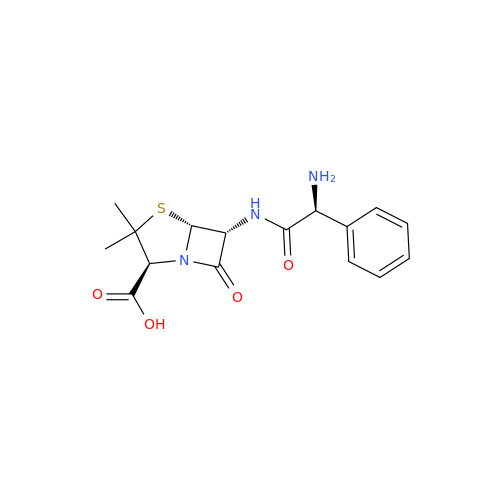
Ampicillin EP Impurity B
|
Chemical Name: Ampicillin EP Impurity B
Synonym: (2S,5R,6R)-6-[[(2S)-2-Amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid| Enter Batch Number | |||