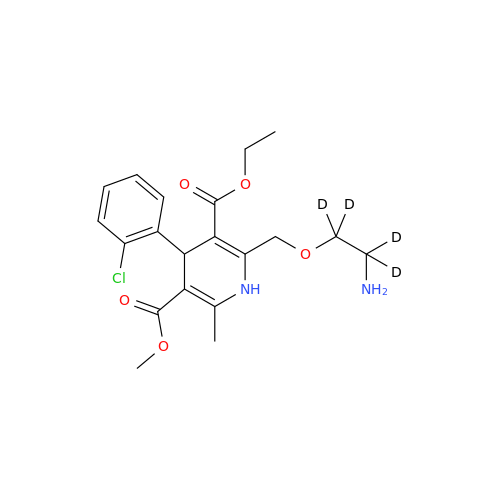
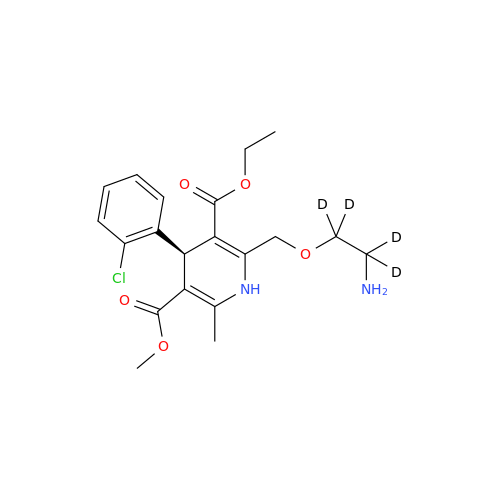
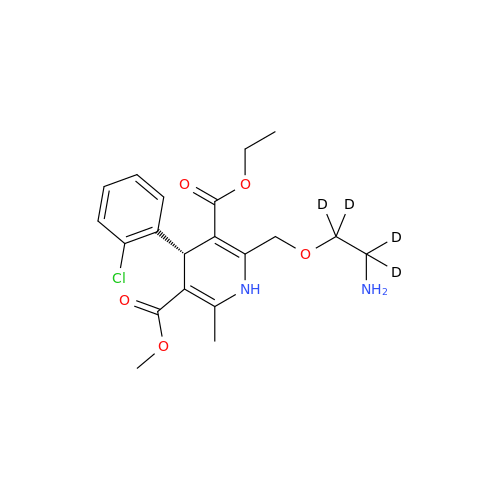
Product Information
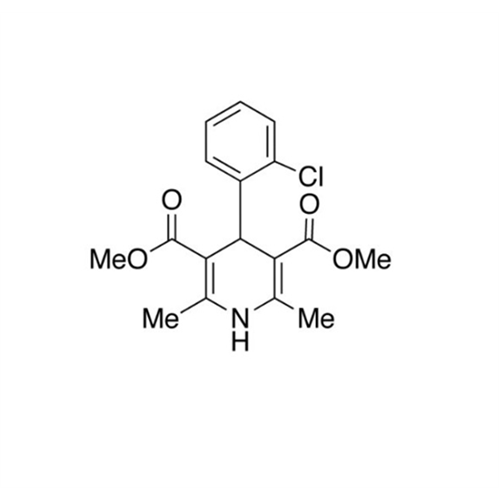
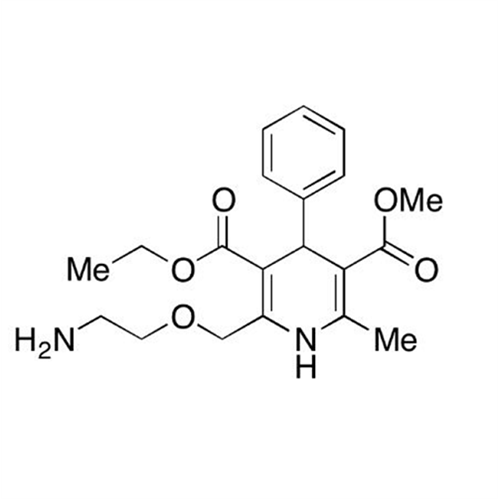
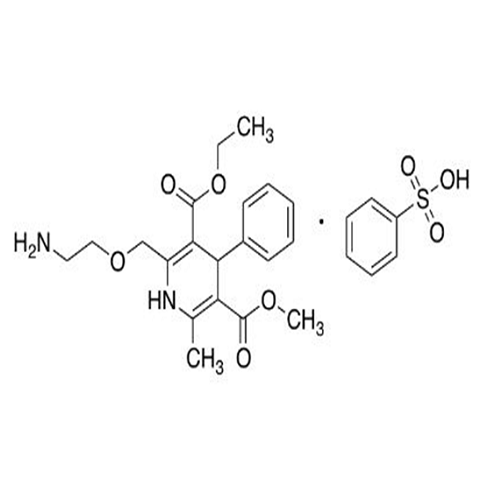
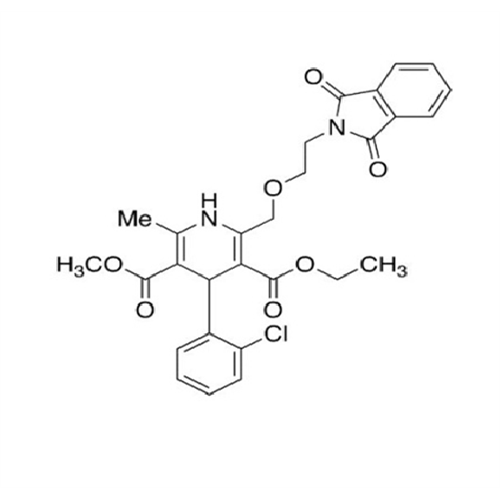
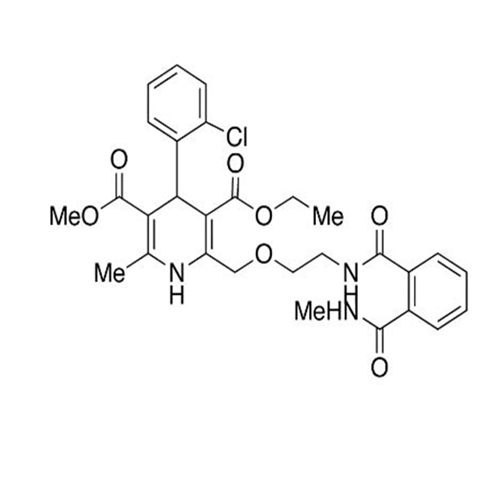
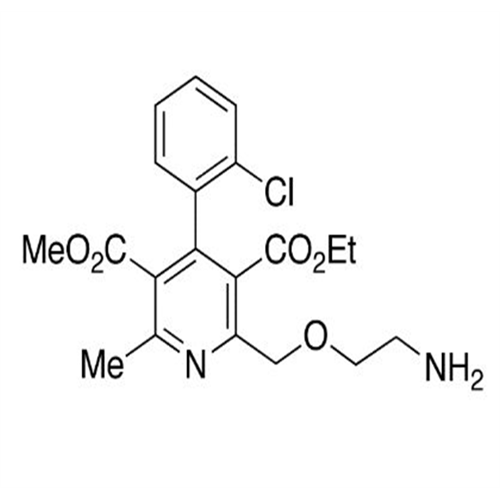
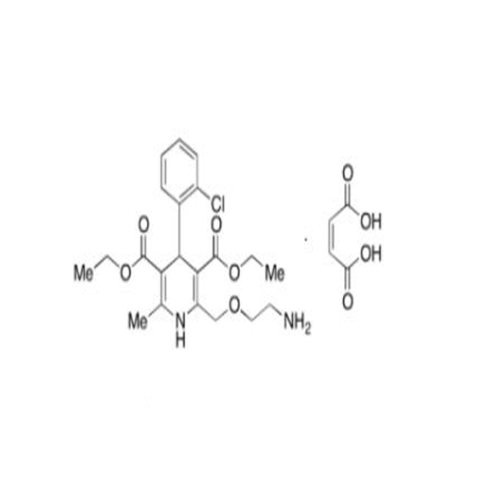
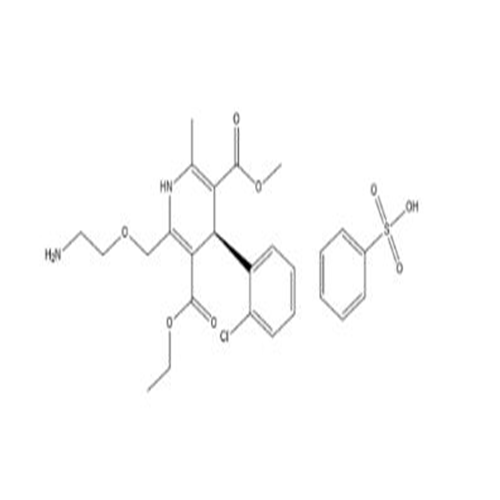
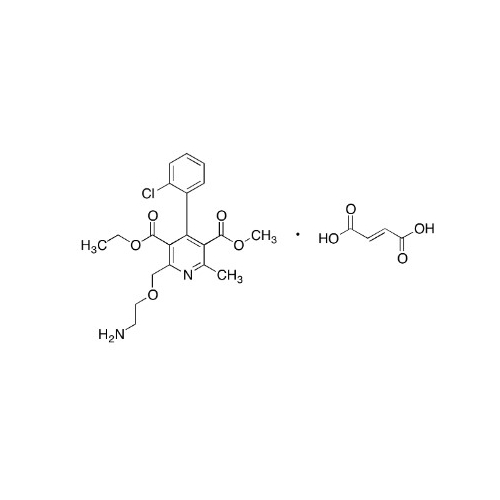
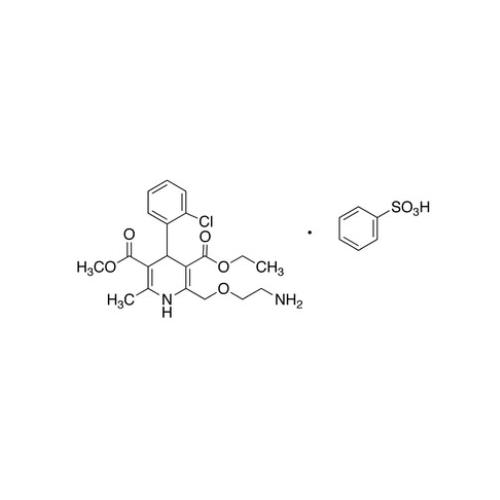
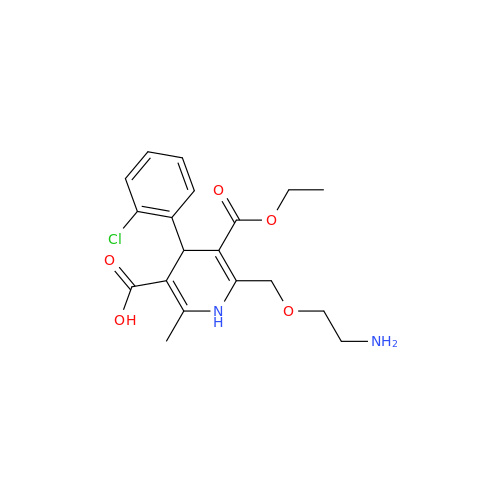
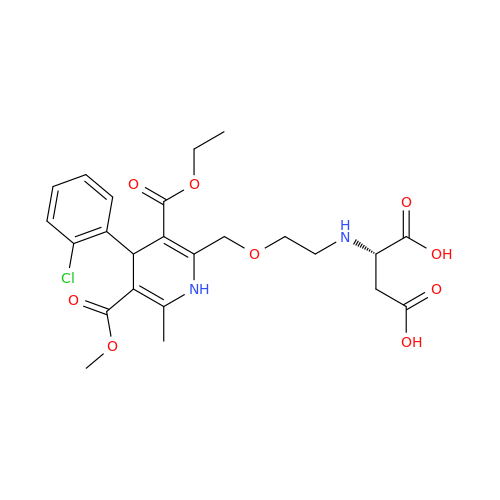
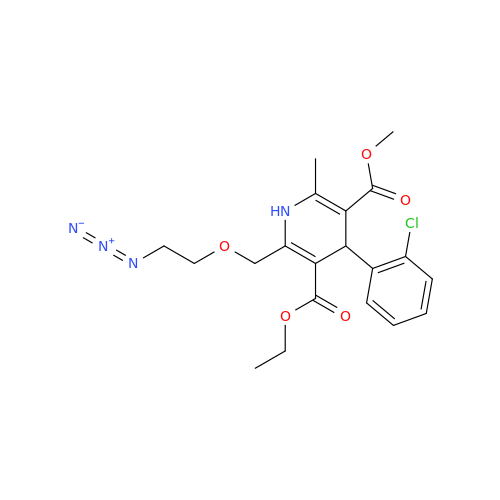
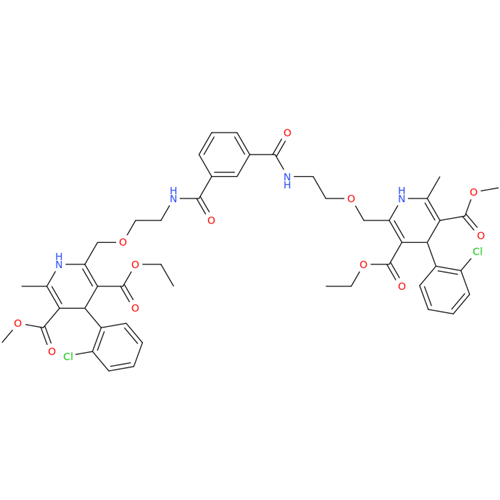
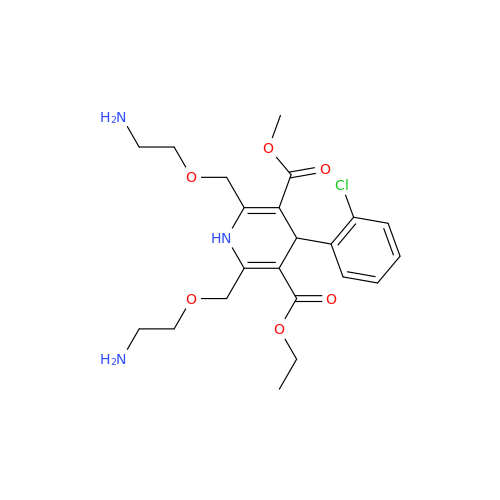
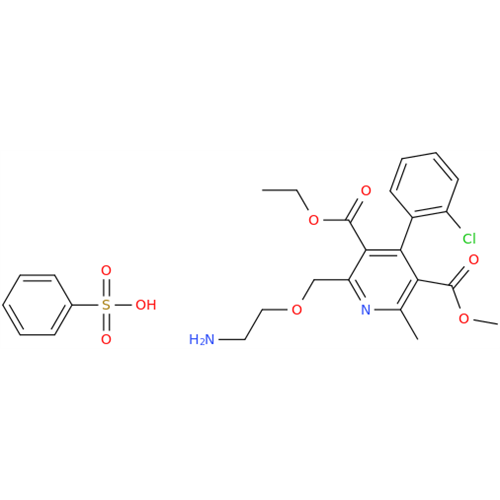
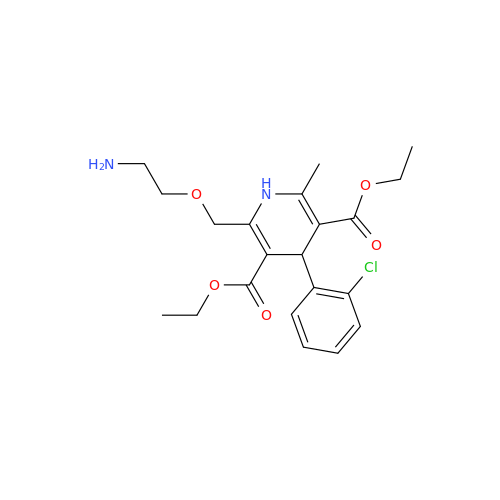
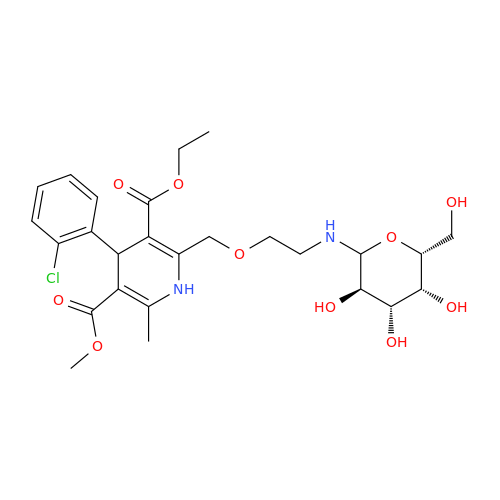
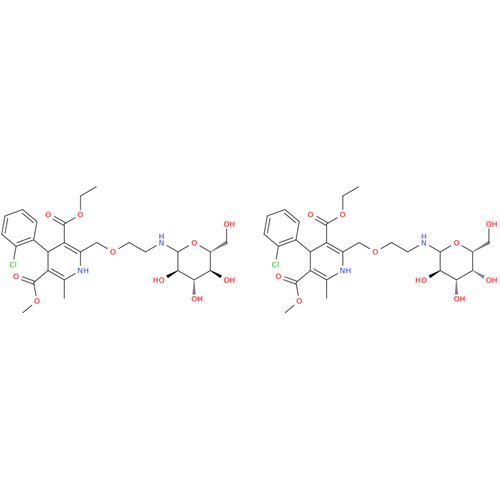
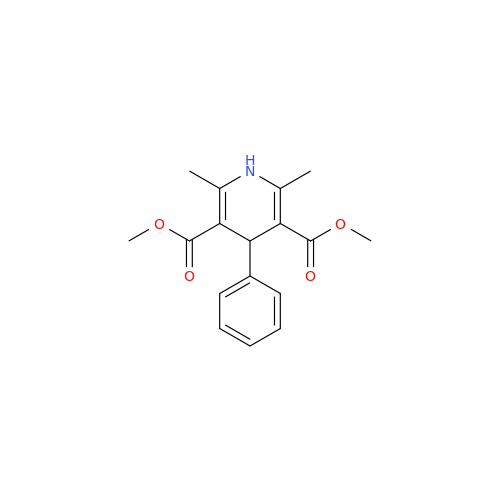
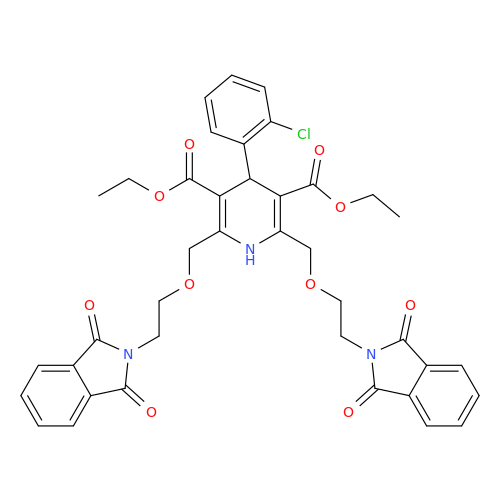
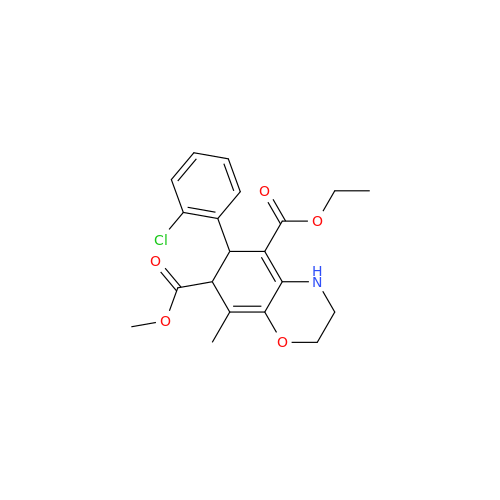
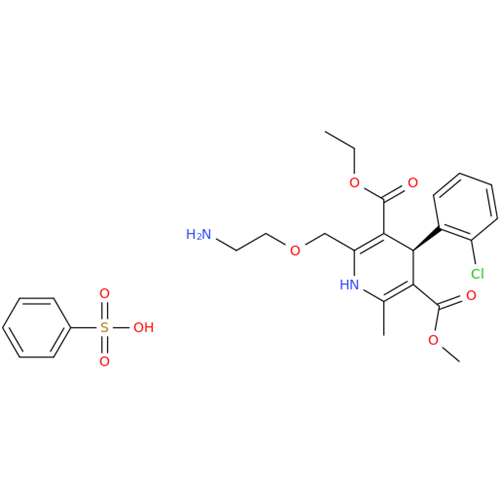
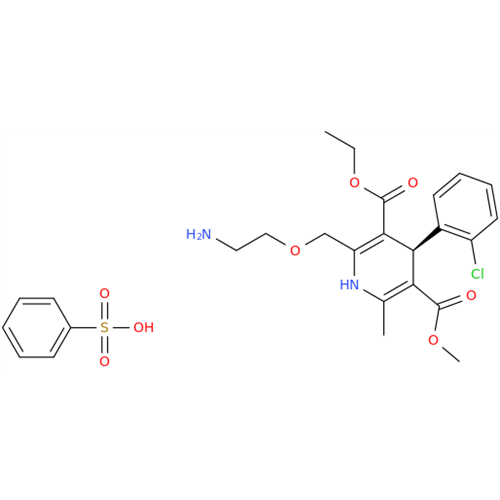
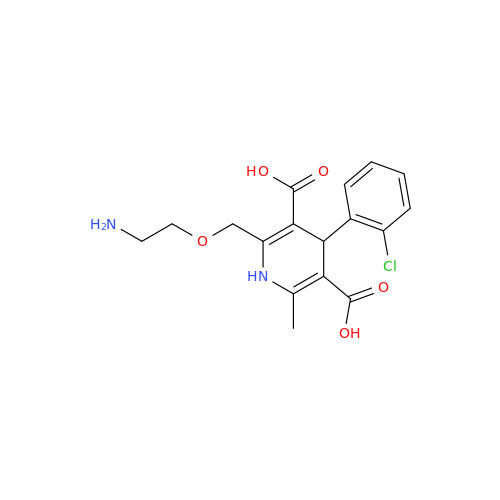
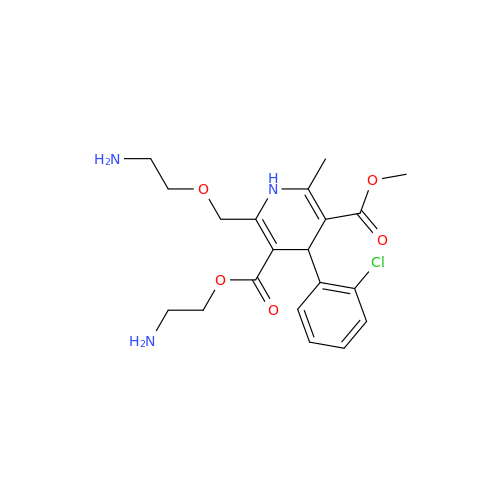
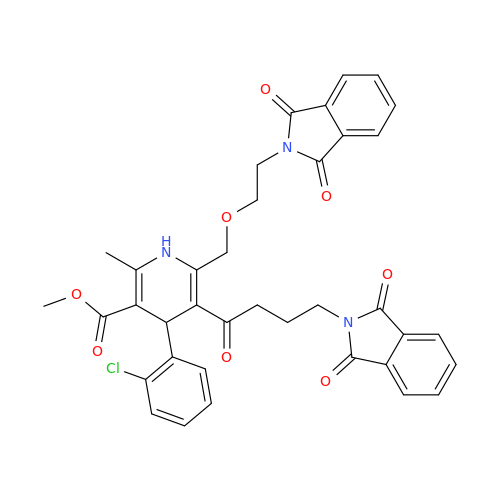
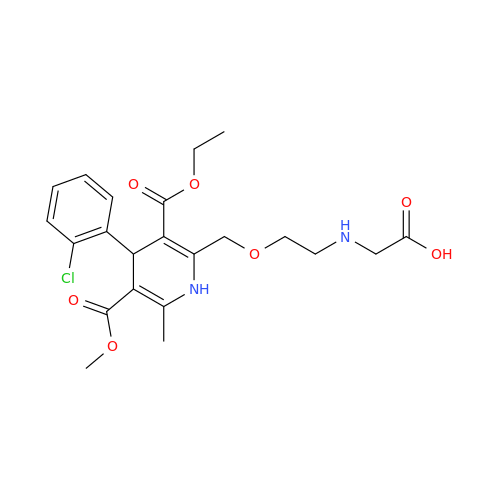
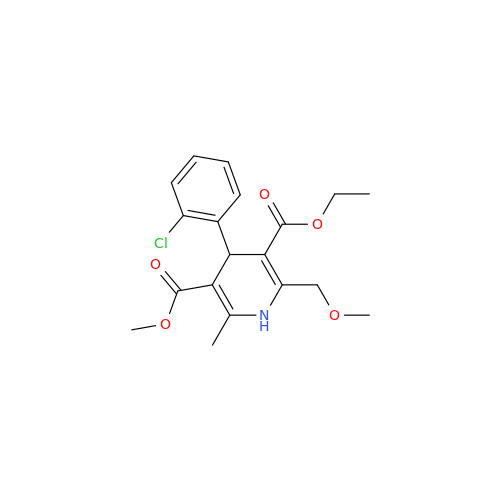
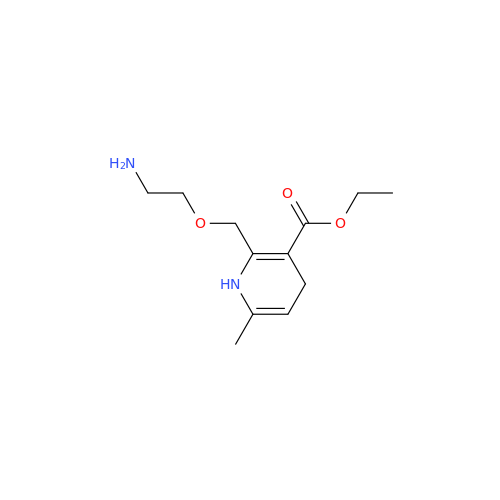
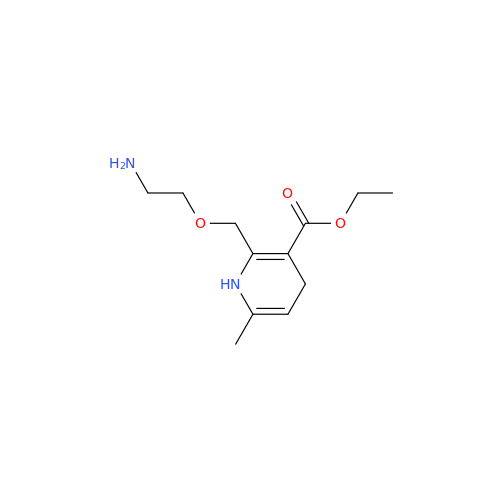
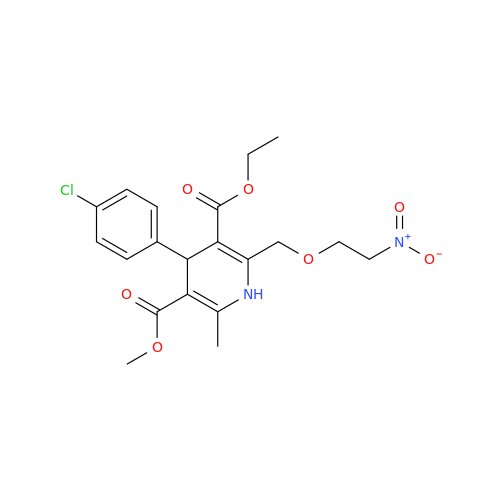
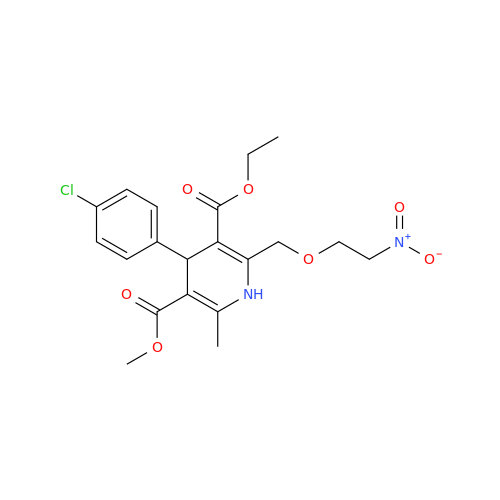
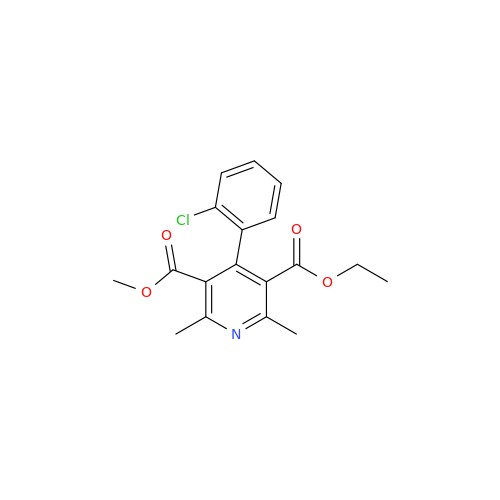
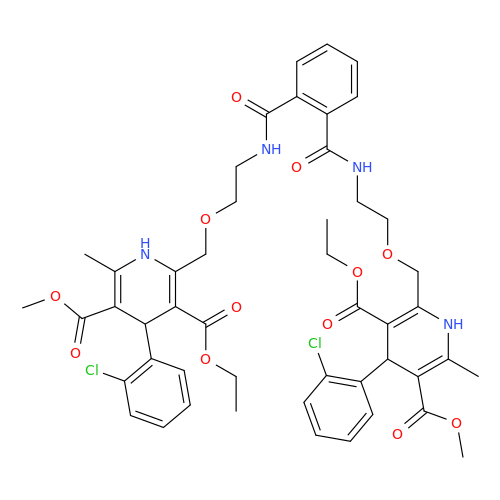
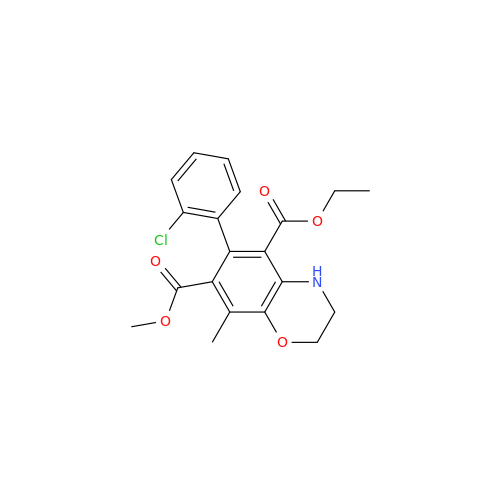
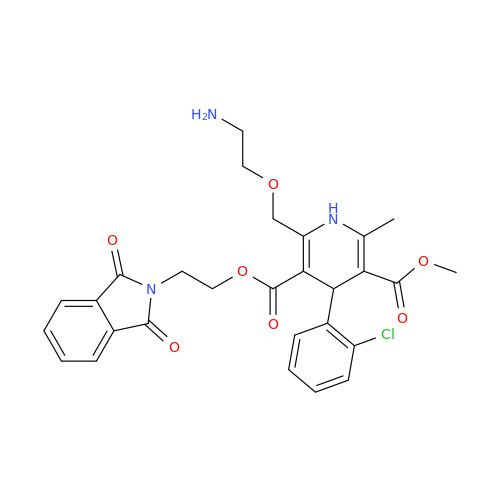
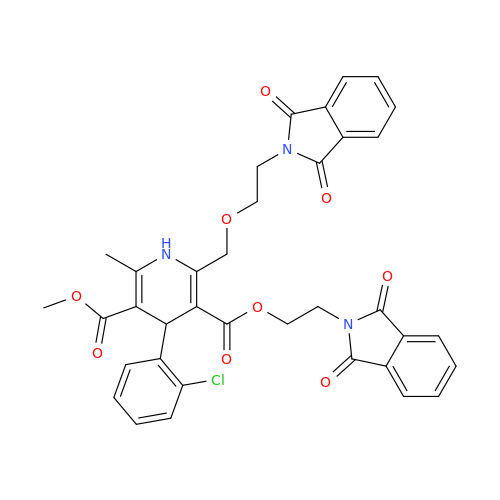
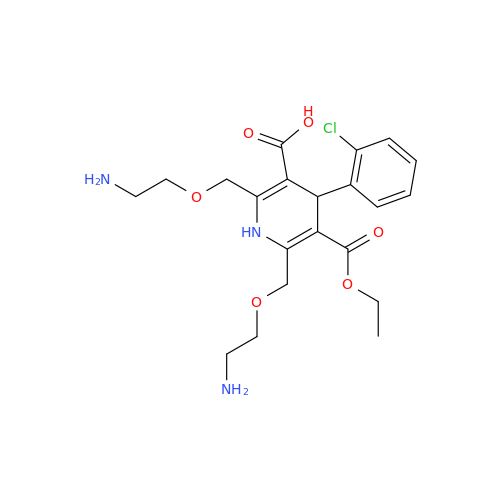
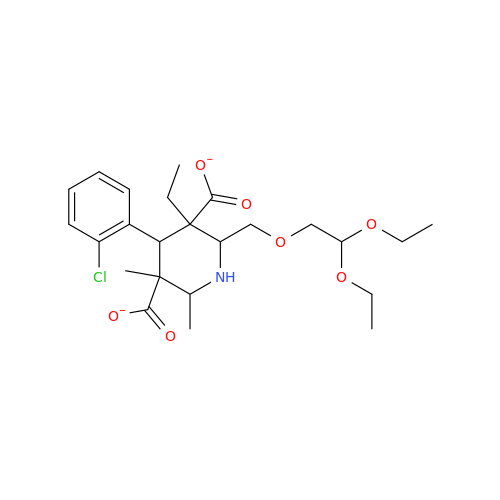
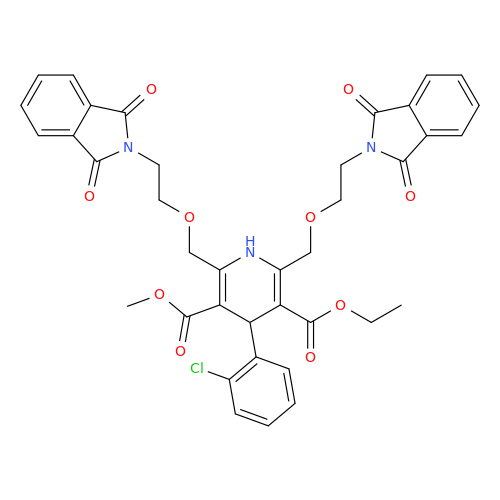
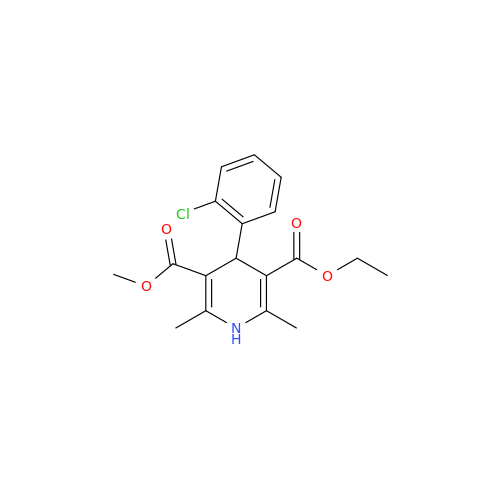
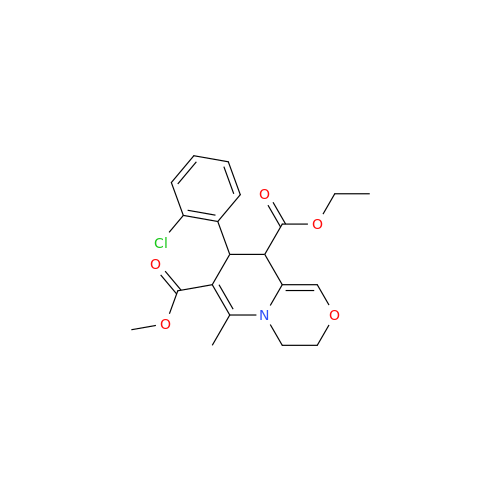
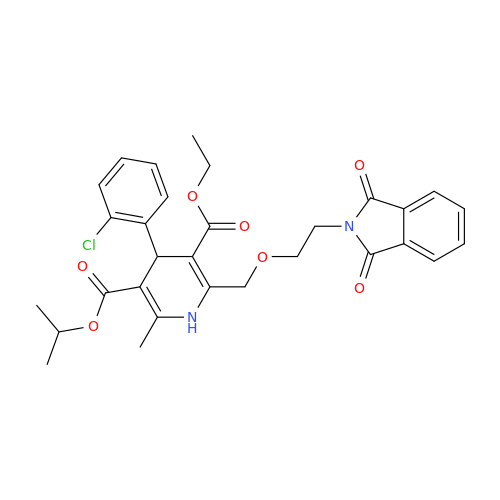
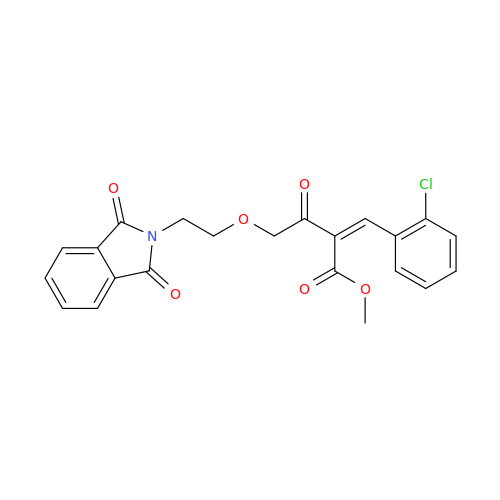
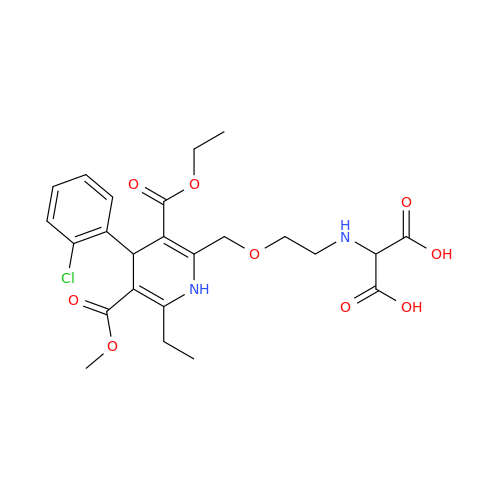
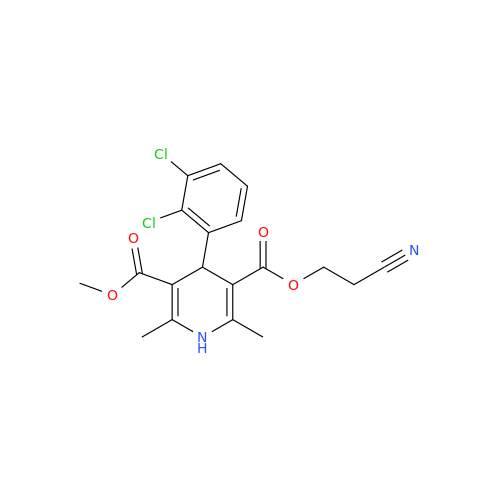
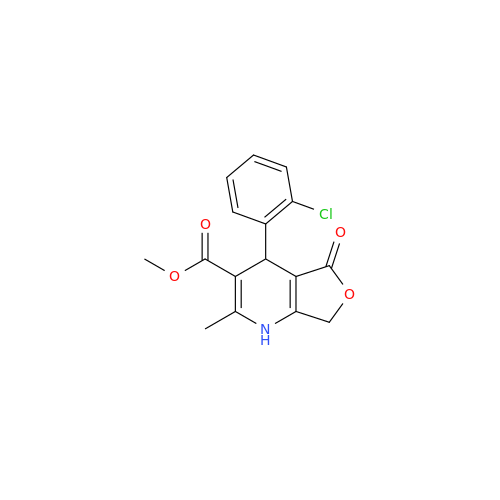
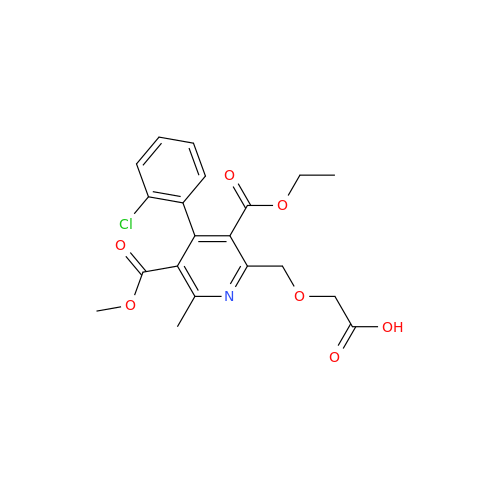
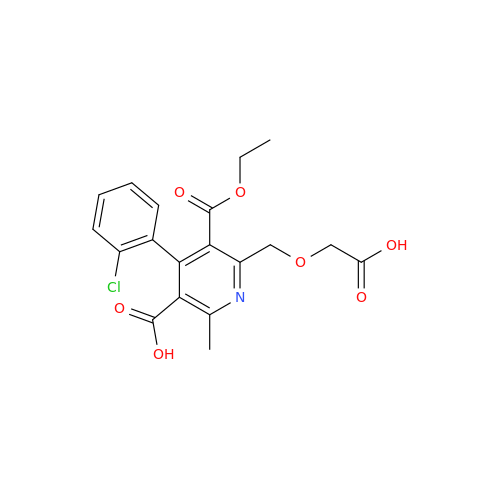
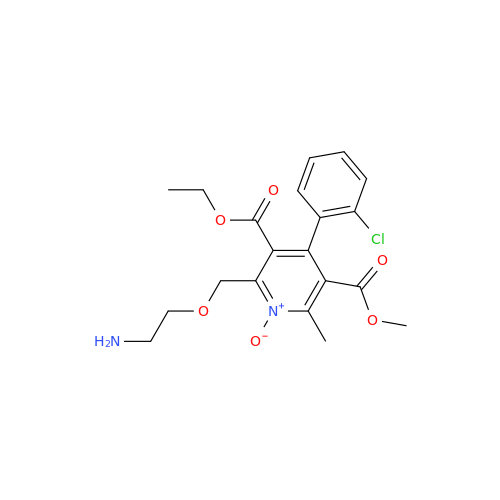
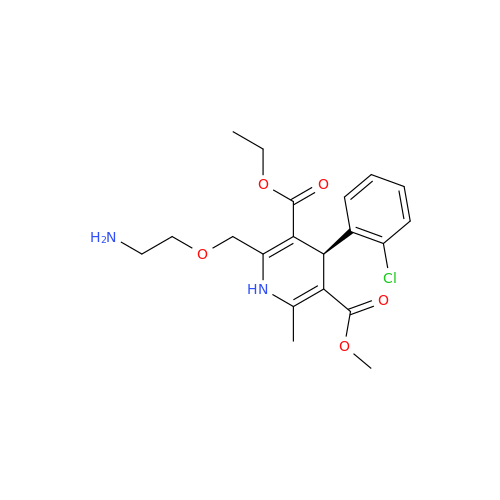
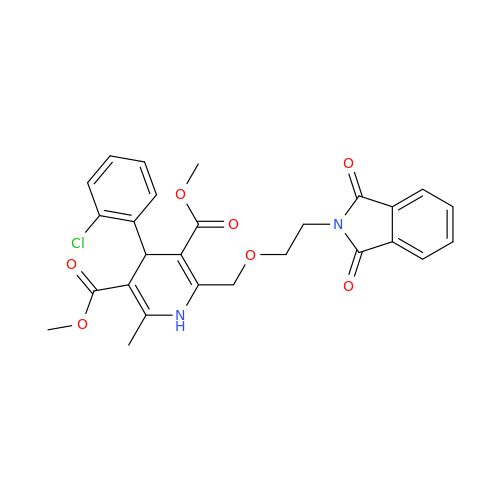
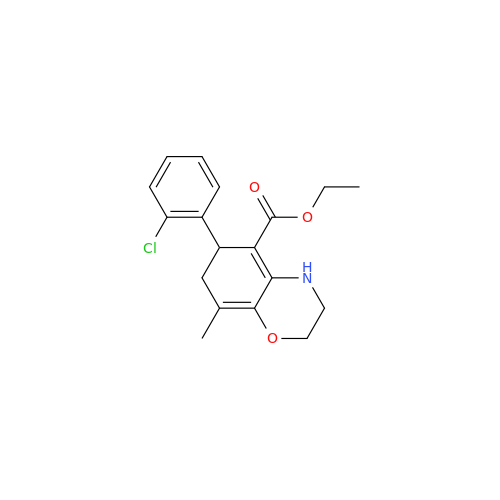
Amlodipine Impurity 48
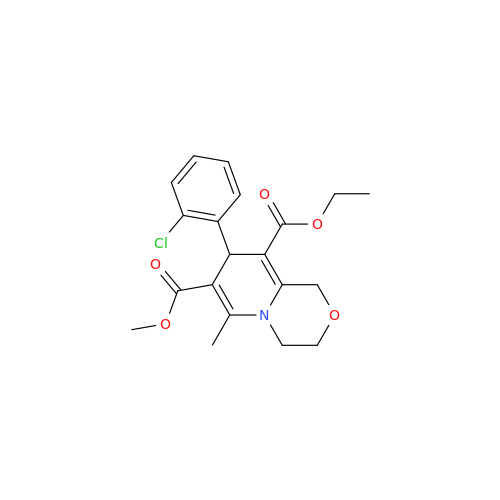
|
Chemical Name: Amlodipine Impurity 48
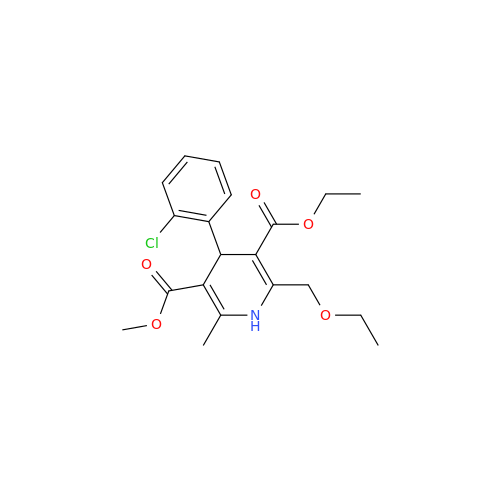
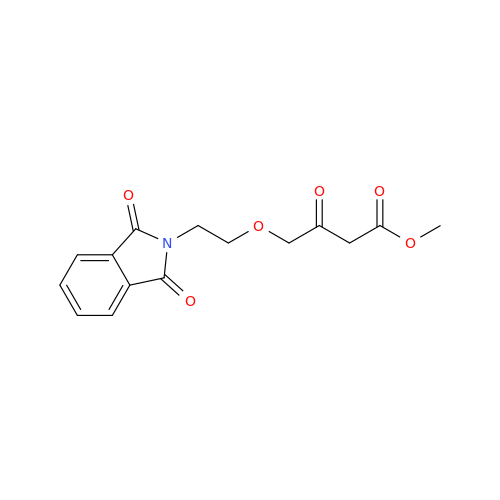
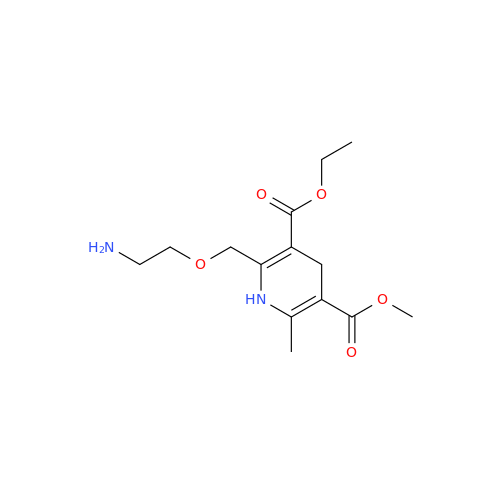
Synonym: 9-Ethyl 7-methyl 8-(2-chlorophenyl)-6-methyl-1,3,4,8-tetrahydropyrido[2,1-c][1,4]oxazine-7,9-dicarboxylate| Enter Batch Number | |||