Product Information
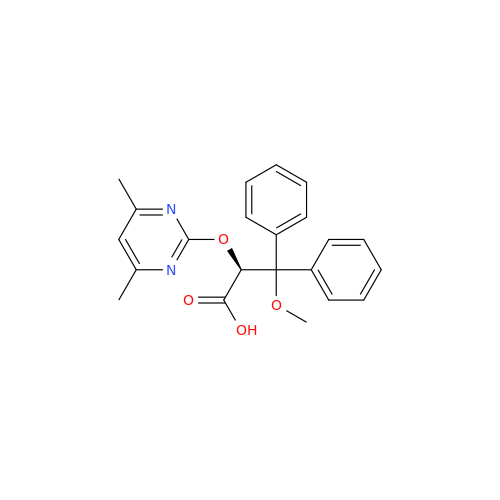
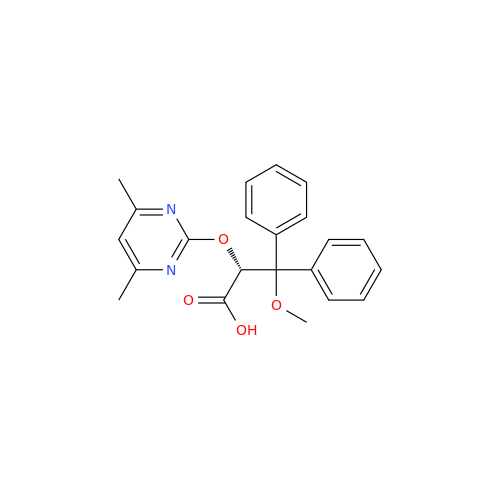
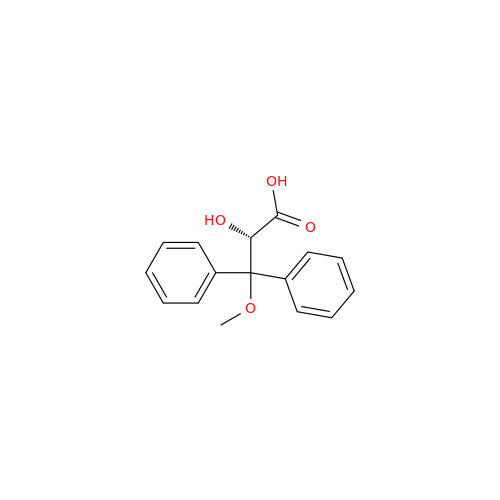
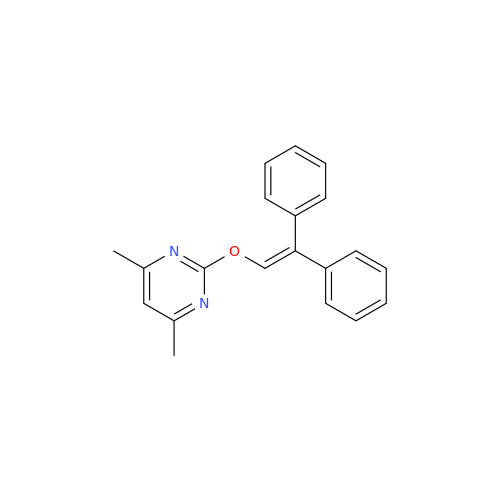
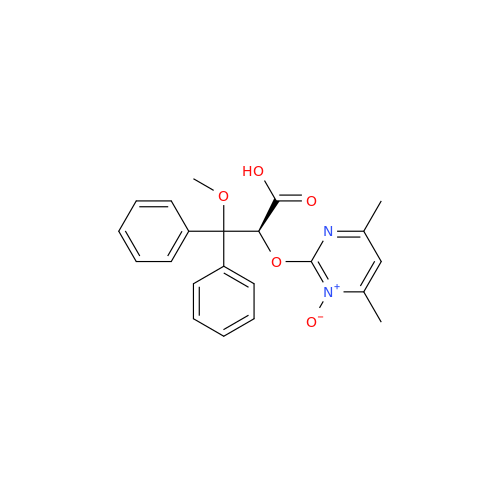
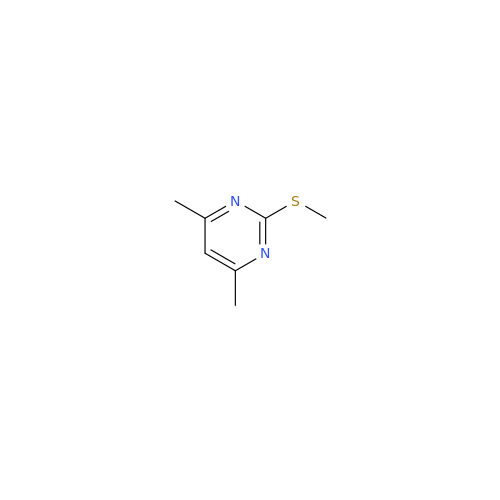
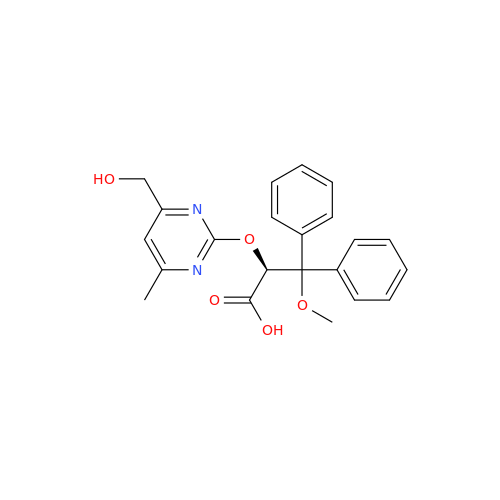
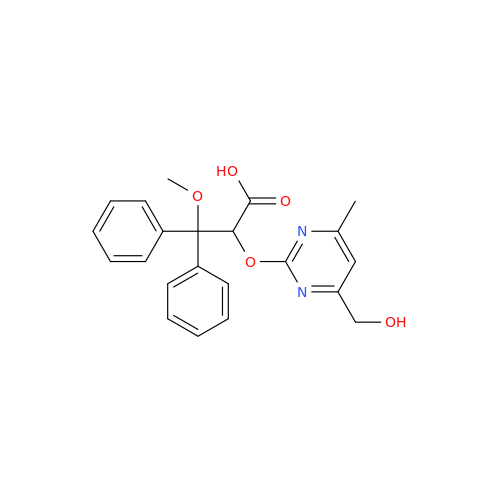
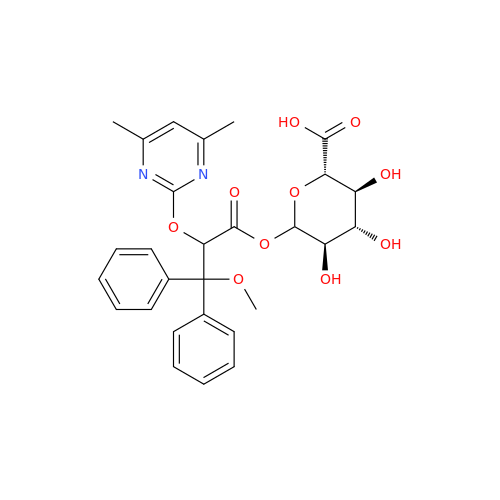
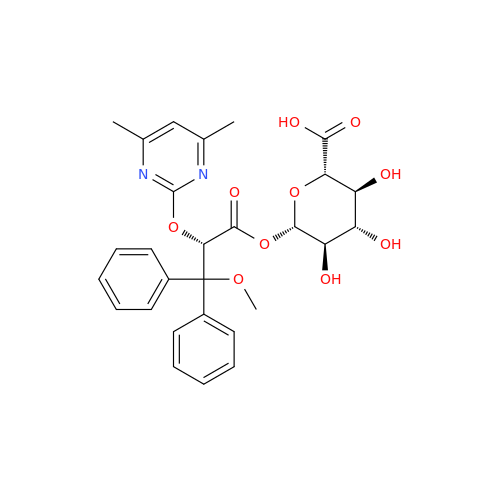
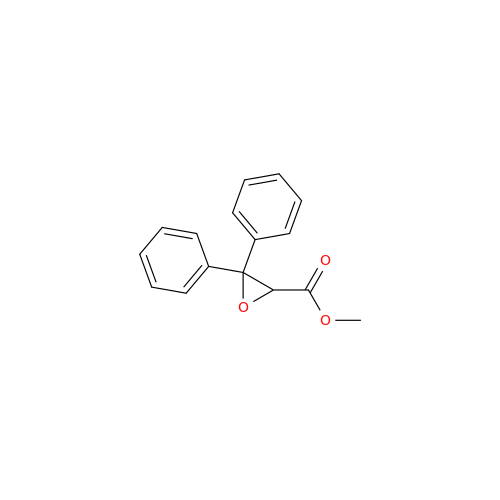
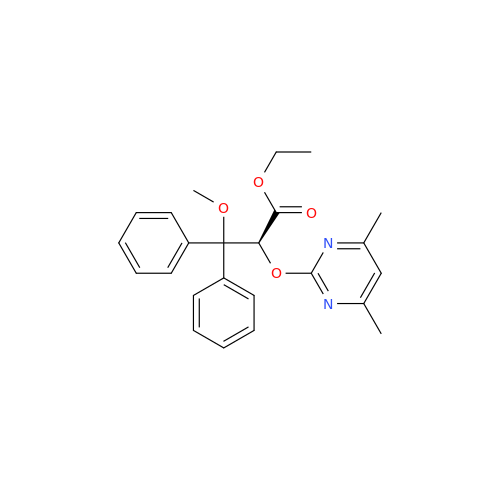
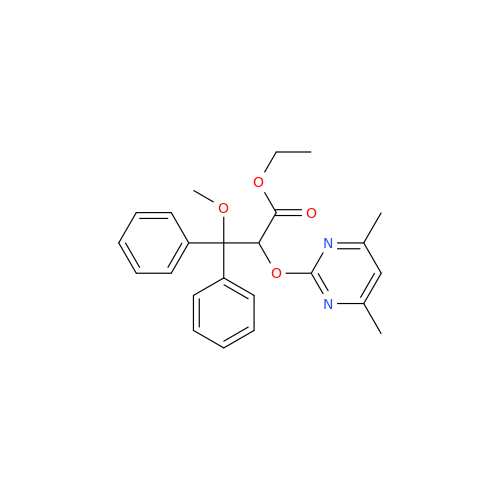
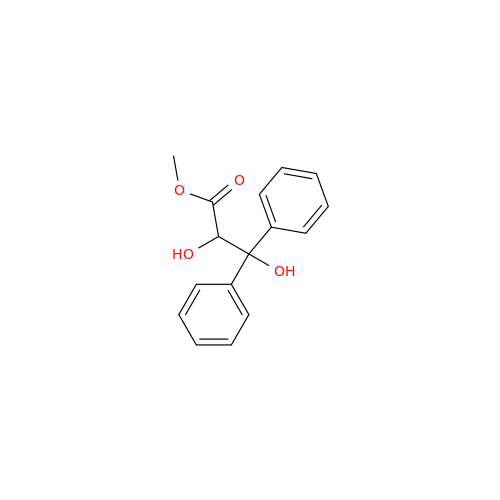
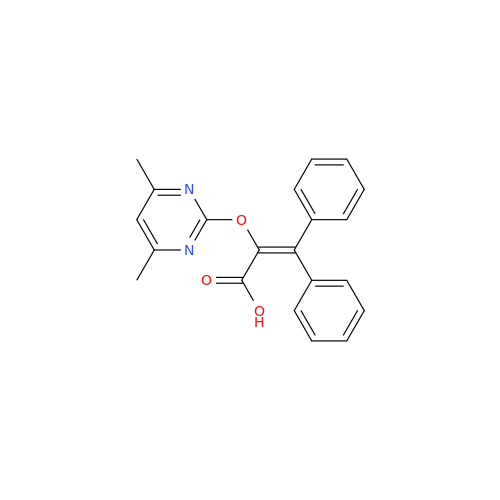
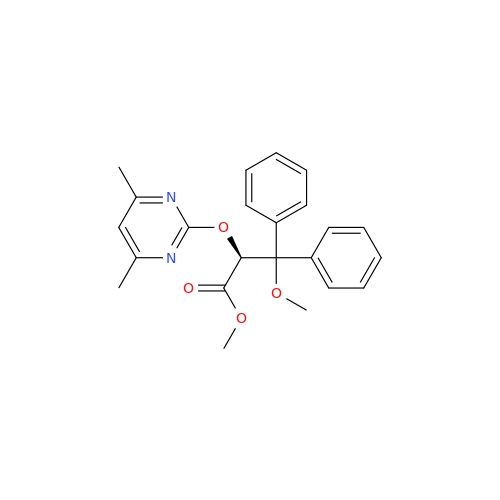
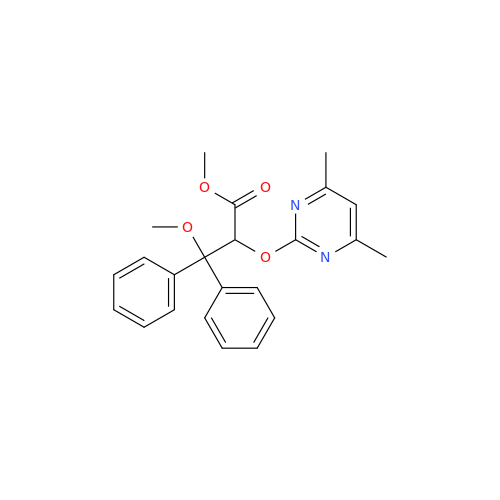
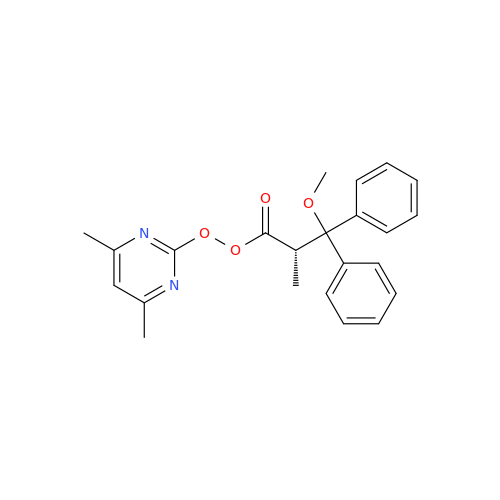
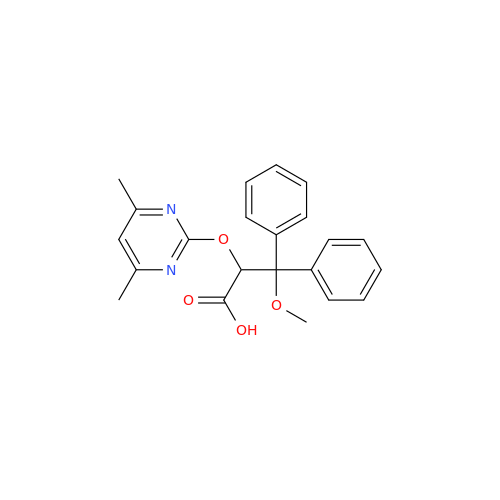
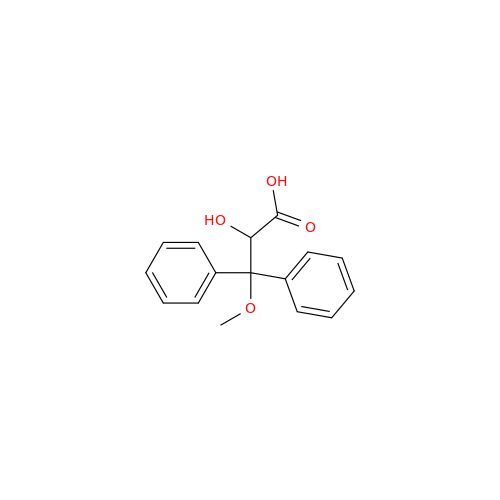
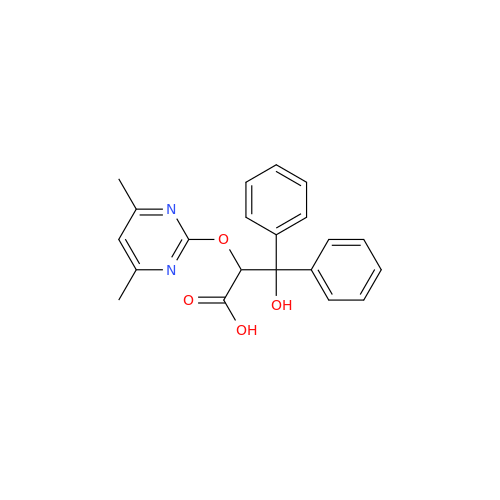
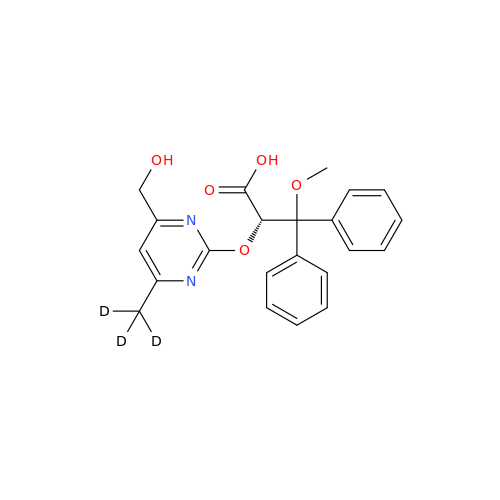
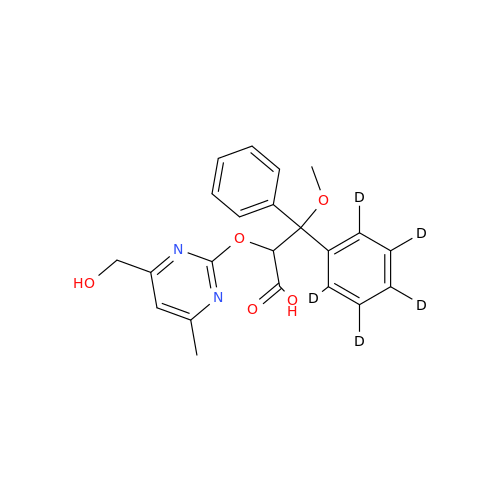
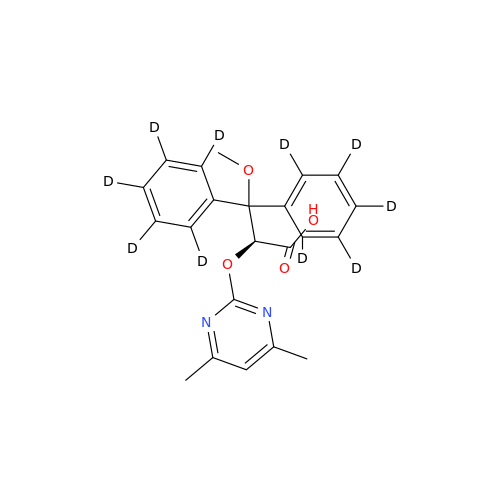
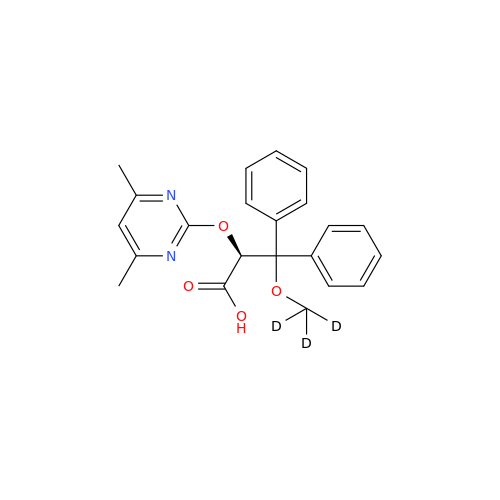
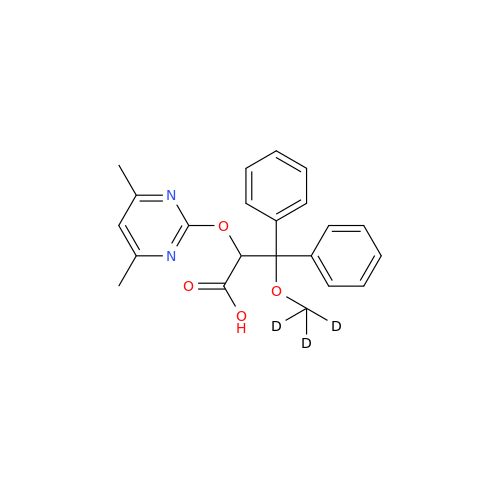
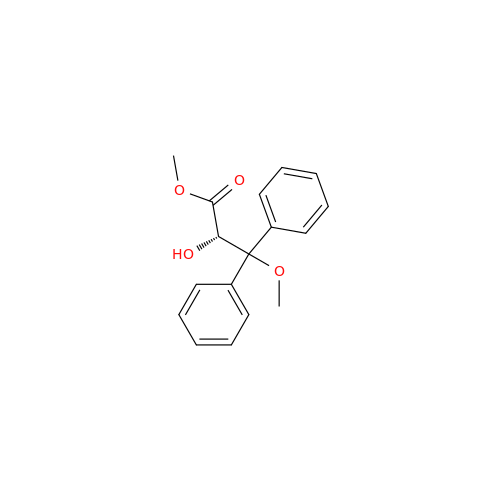
Ambrisentan Hydroxyester Impurity
|
Chemical Name: Ambrisentan Hydroxyester Impurity
Synonym: 3-Diphenylpropanoate, (S)-methyl 2-hydroxy-3-methoxy-3; Ambrisentan Hydroxyester Impurity| Enter Batch Number | |||