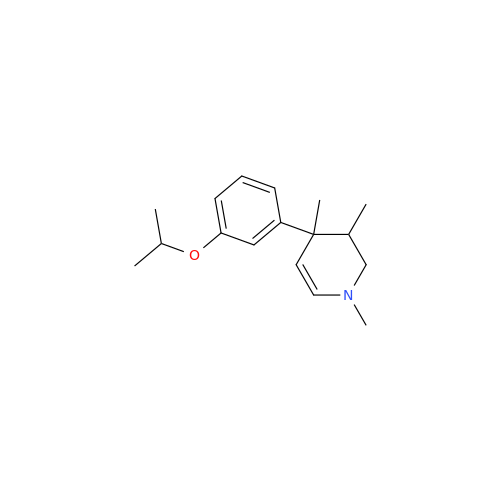
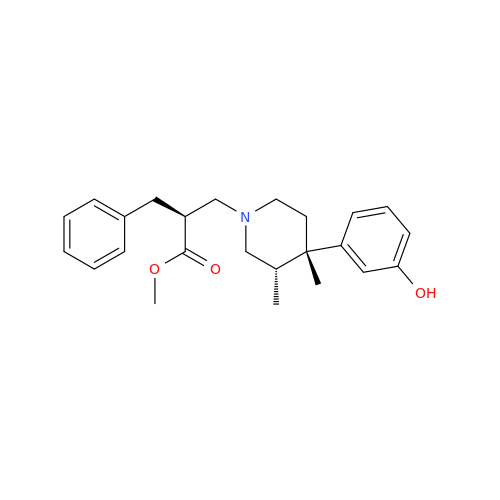
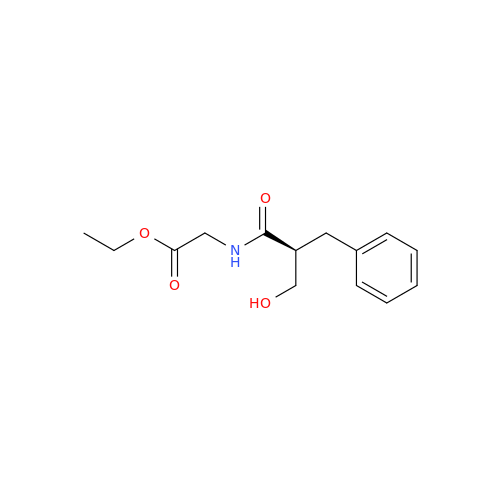
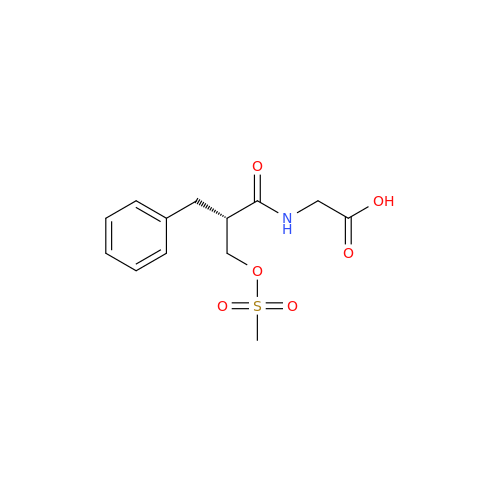
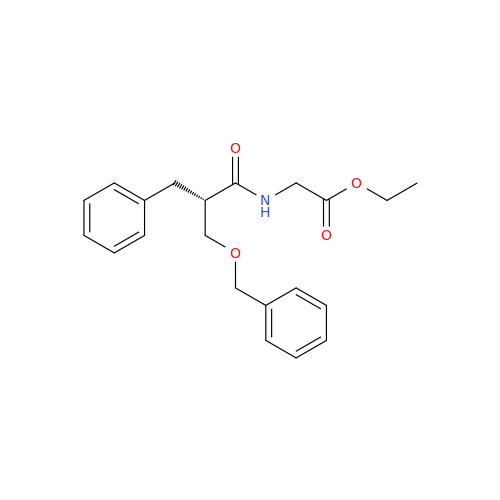
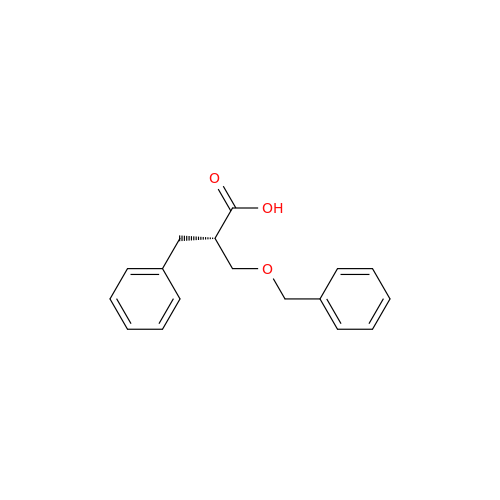
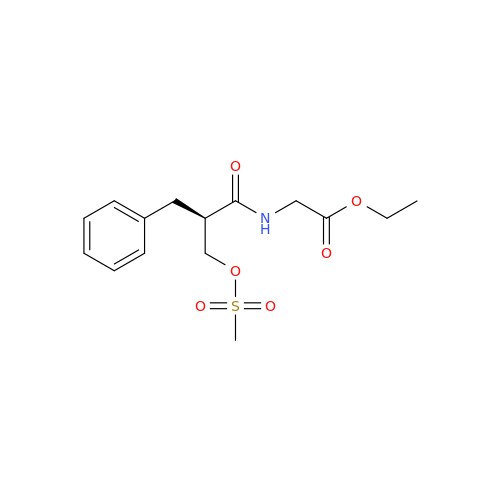
Product Information
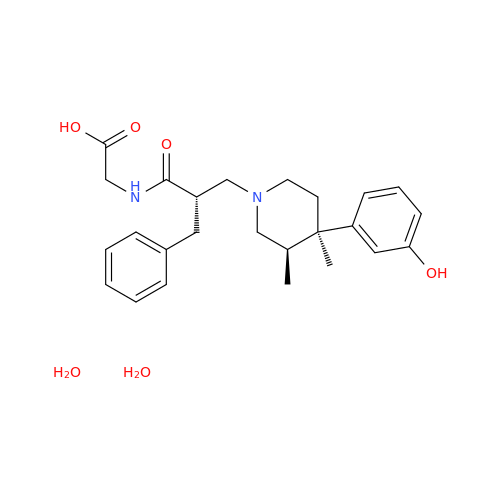
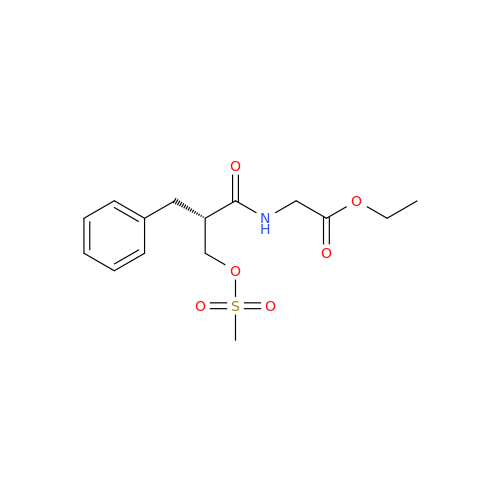
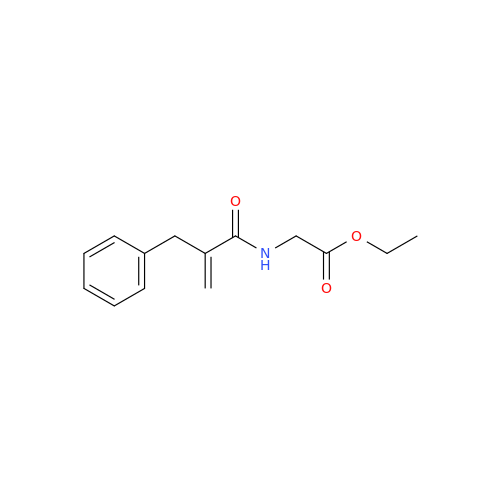
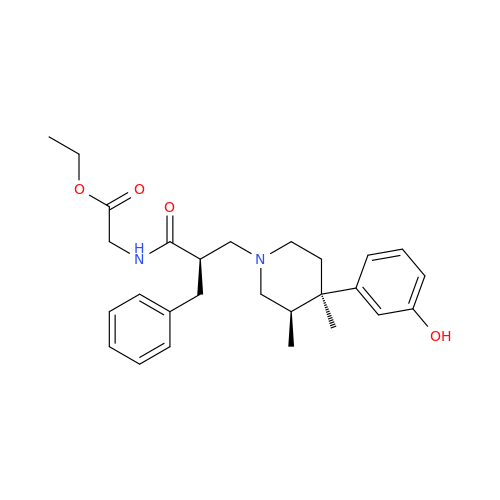
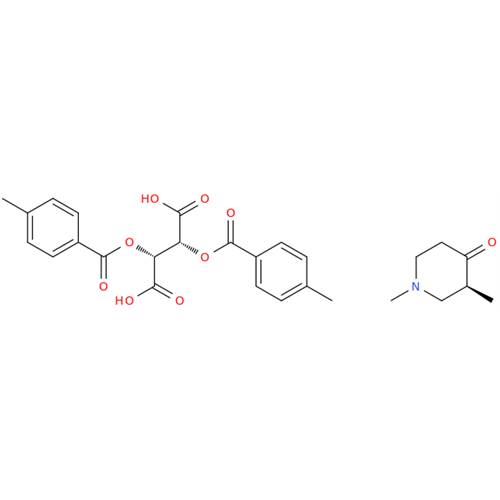
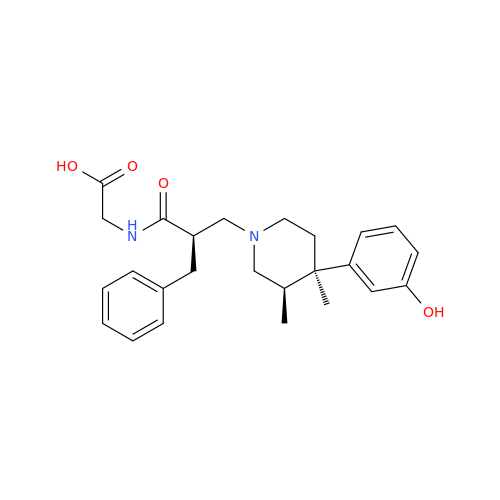
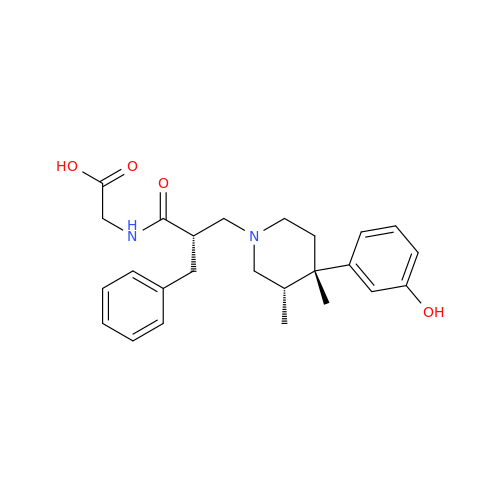
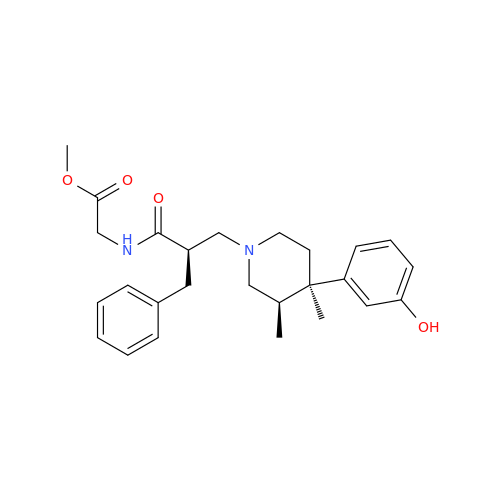
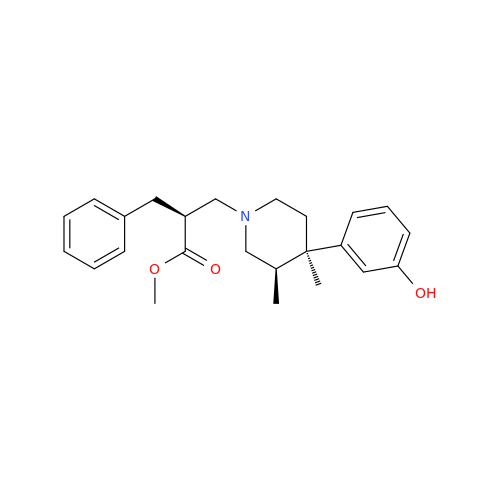
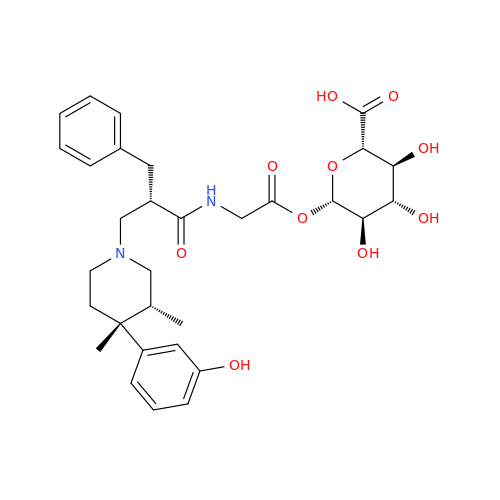
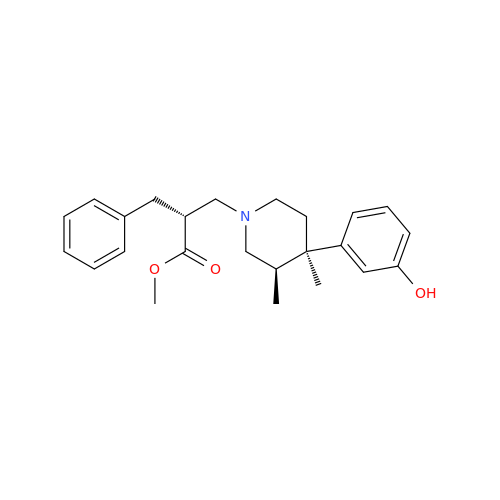
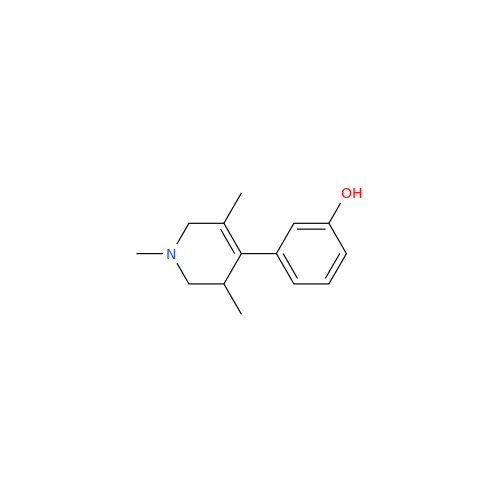
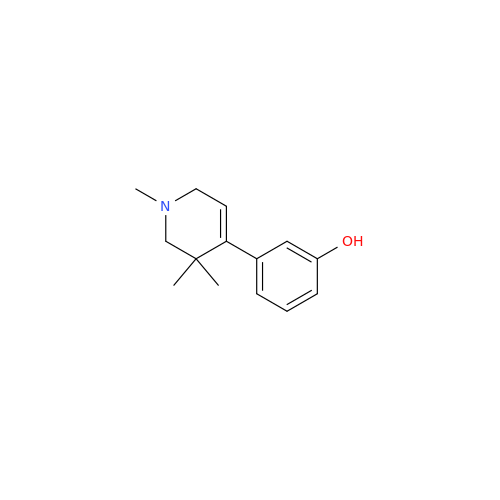
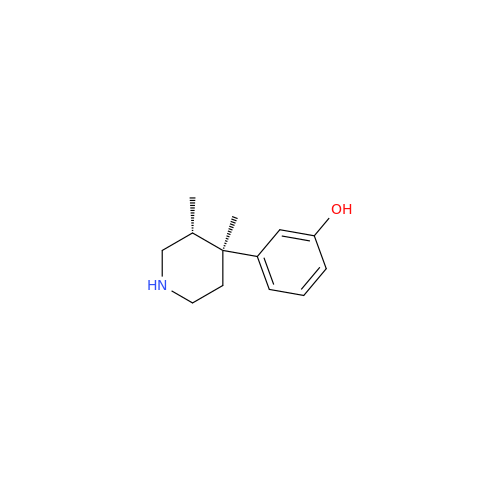
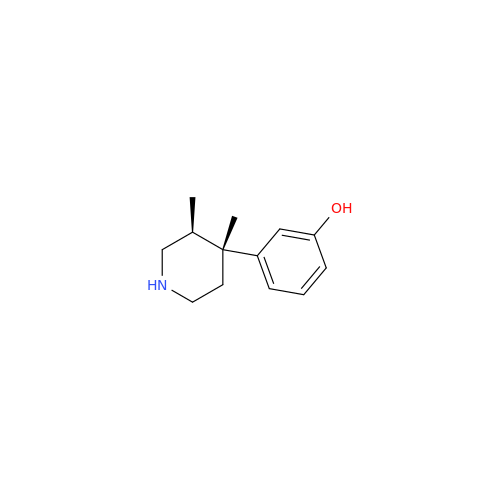
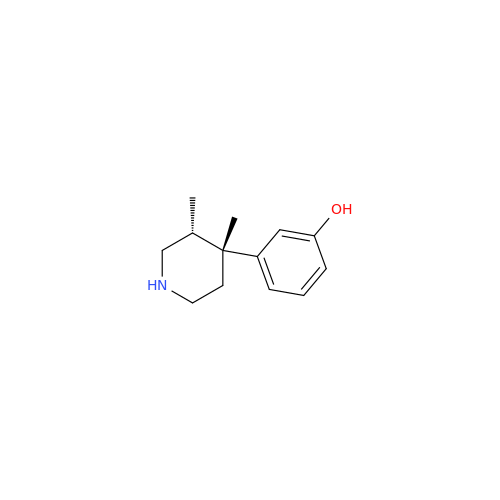
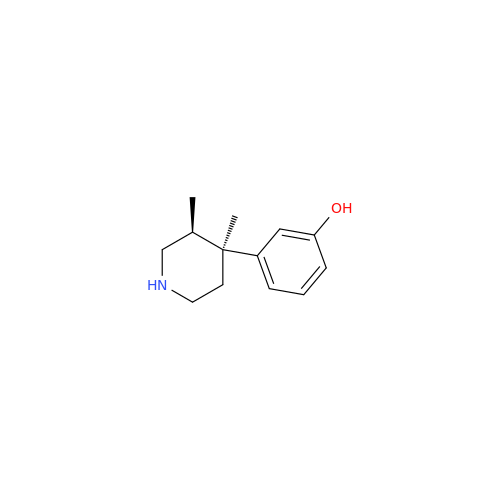
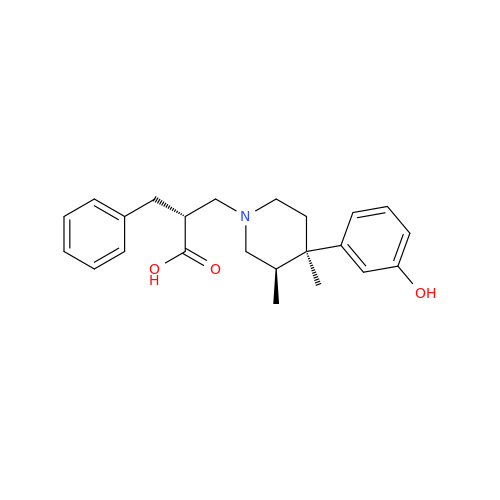
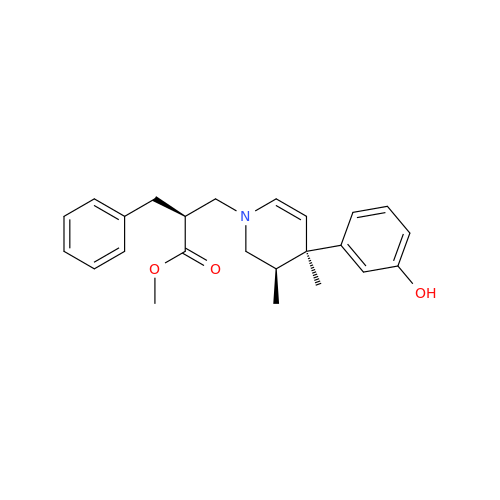
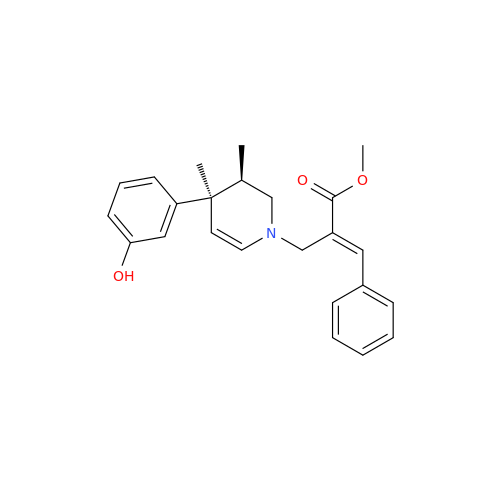
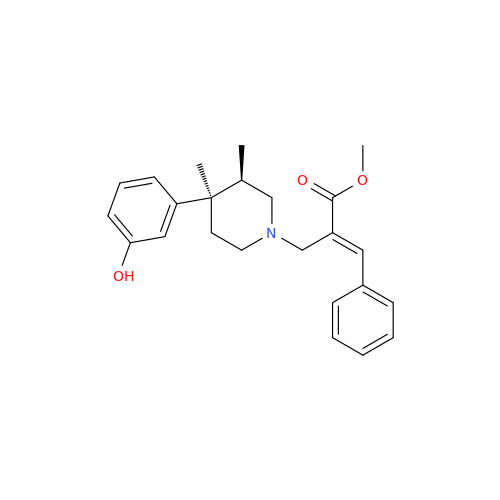
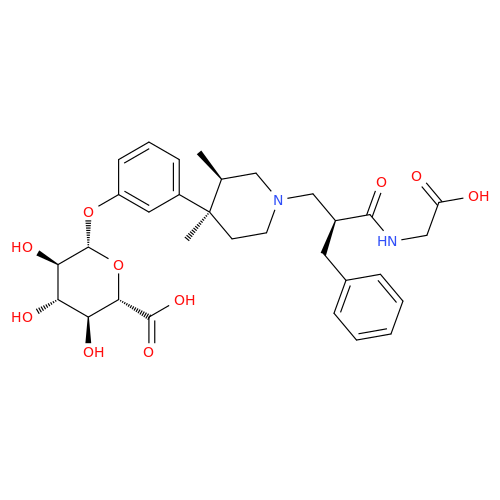
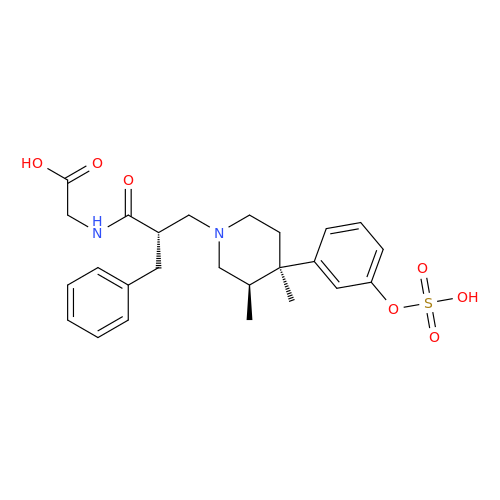
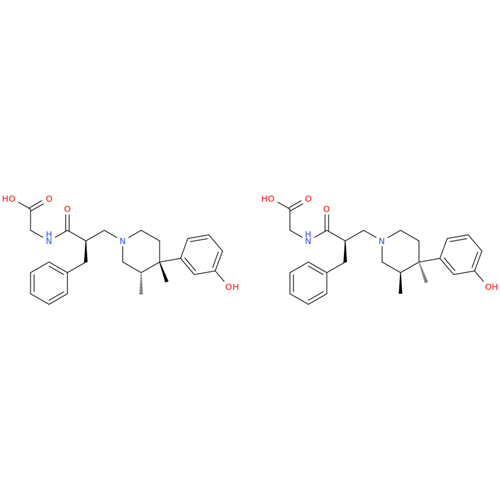
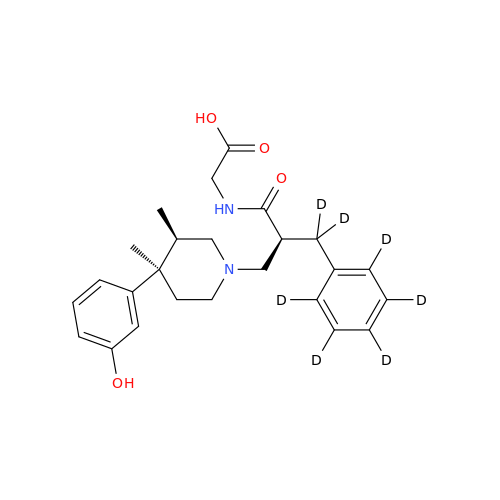
Alvimopan Impurity 9
|
Chemical Name: Alvimopan Impurity 9
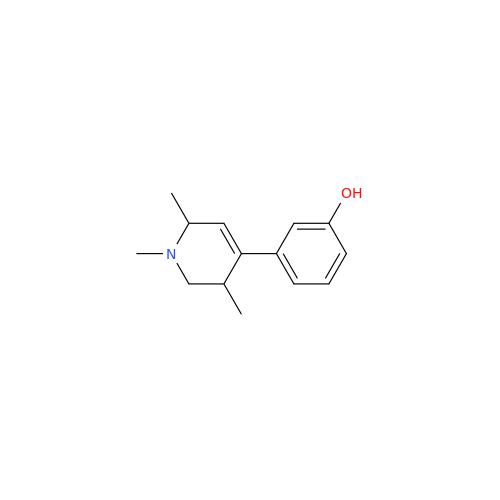
Synonym: 3-(1,3,6-Trimethyl-1,2,3,6-tetrahydropyridin-4-yl)phenol| Enter Batch Number | |||