Product Information
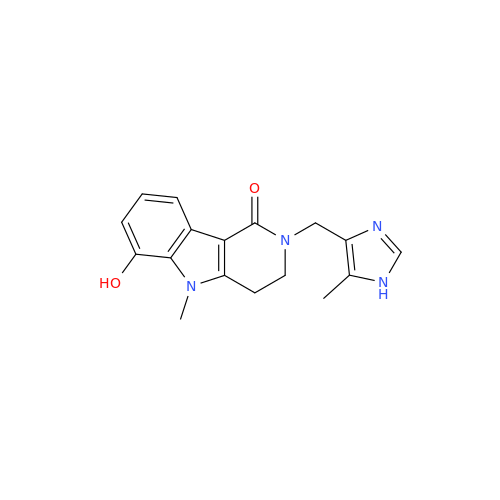
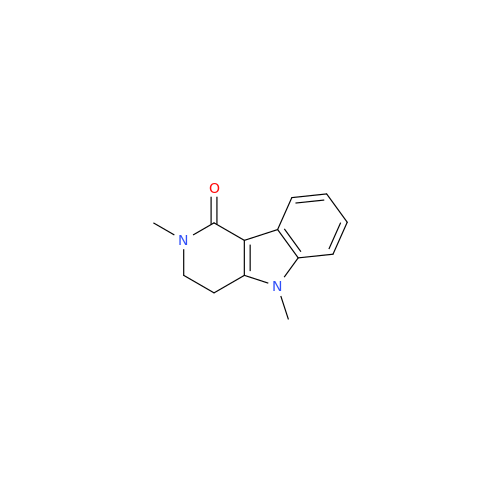
Alosetron Impurity 1
|
Chemical Name: Alosetron Impurity 1
Synonym: 2,5-Dimethyl-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indol-1-one| Enter Batch Number | |||