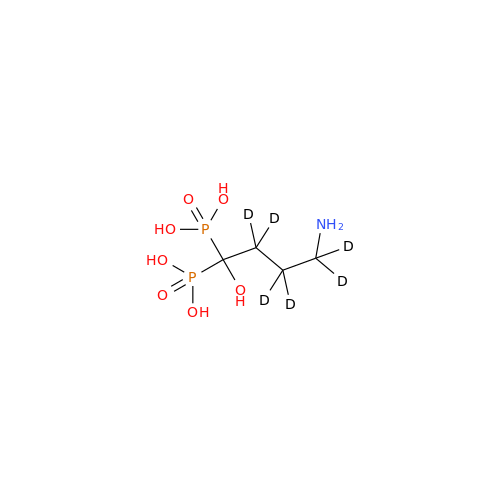
Product Information
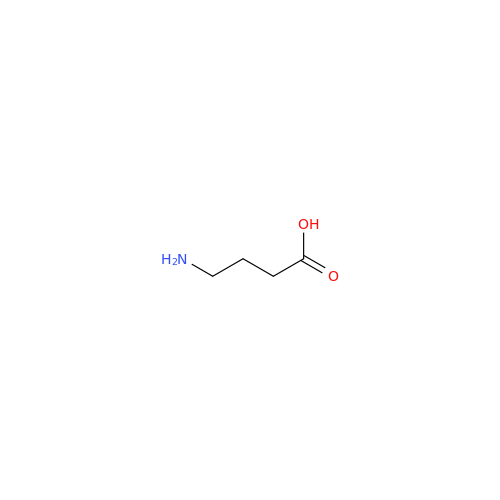
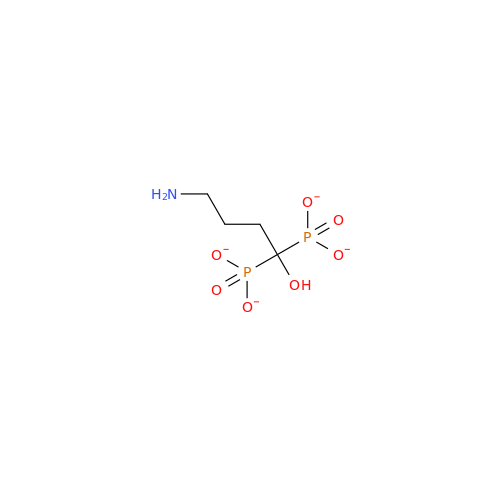
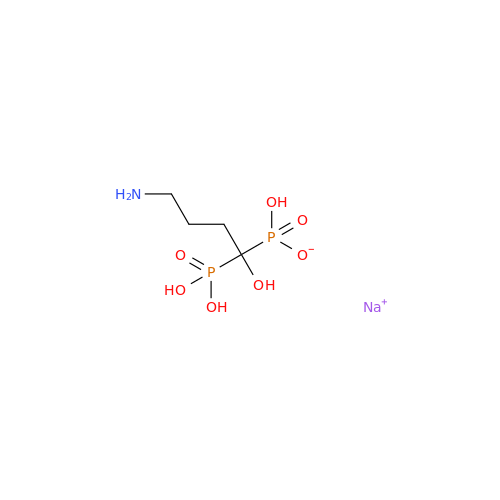
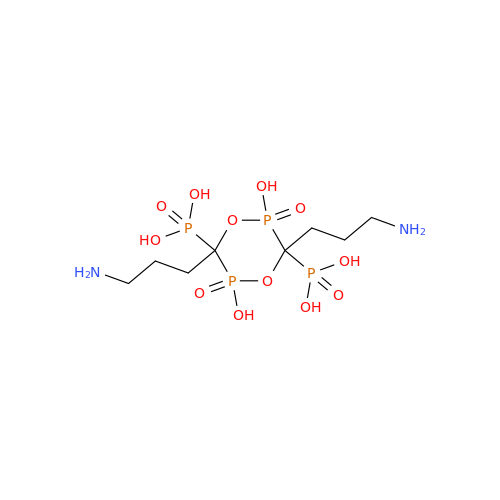
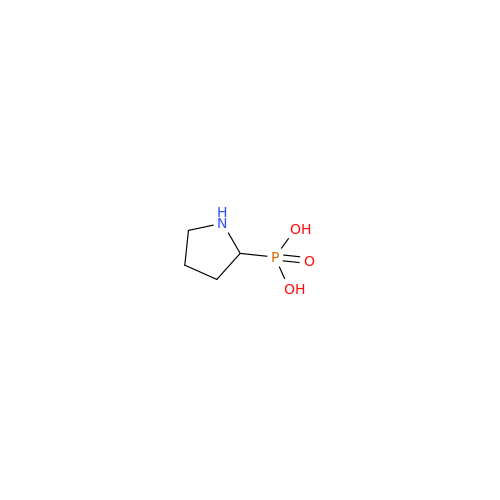
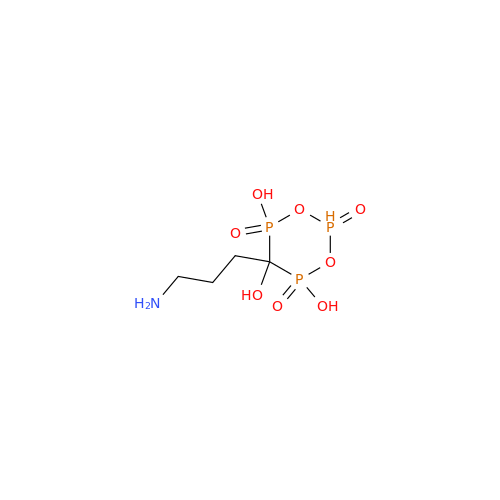
Alendronate EP Impurity B
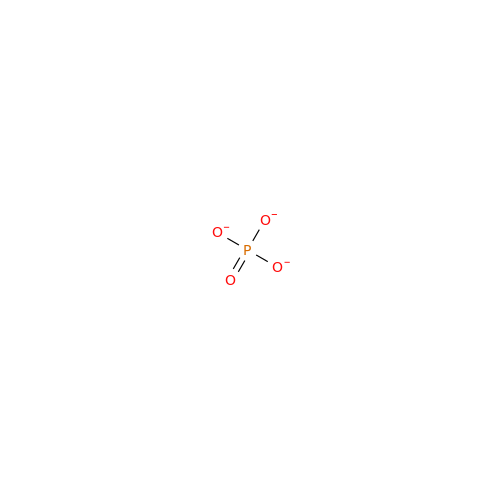
|
Chemical Name: Alendronate EP Impurity B
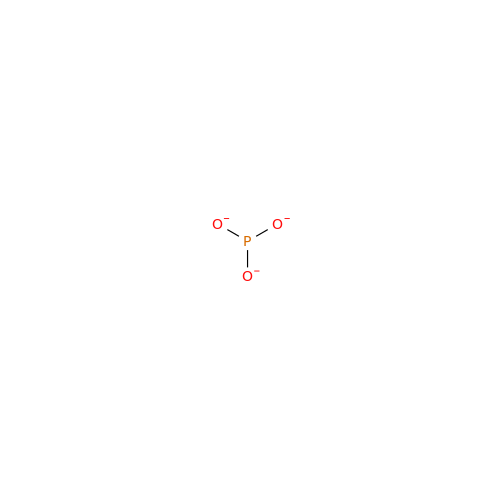
Synonym: Orthophosphate; Phosphate anion; Phosphate ion; Phosphate trianion; Phosphate(3-)| Enter Batch Number | |||