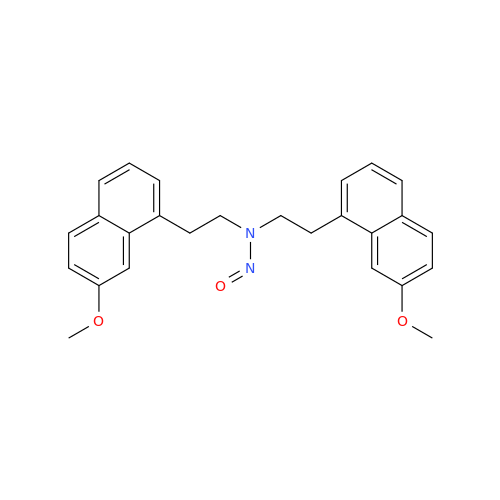
Product Information
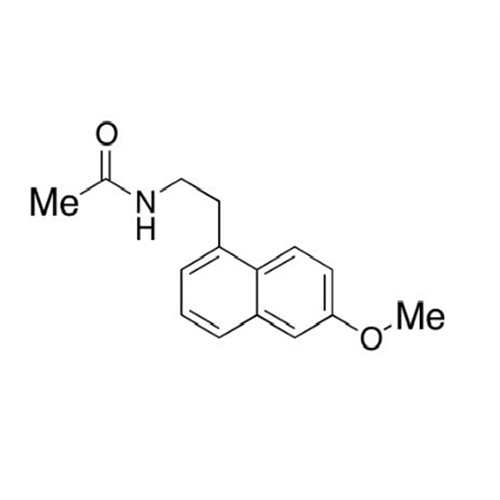
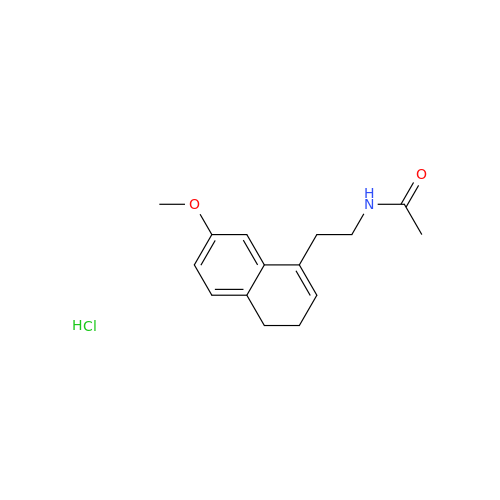
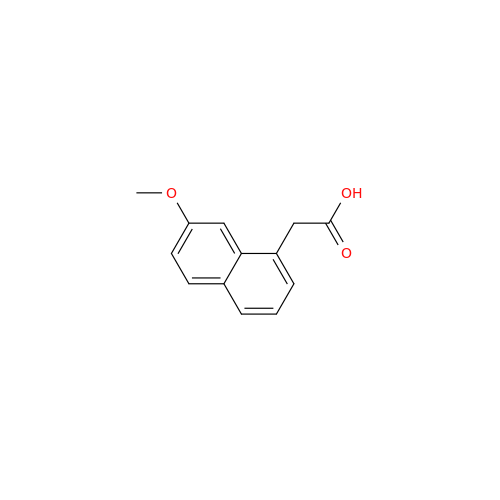
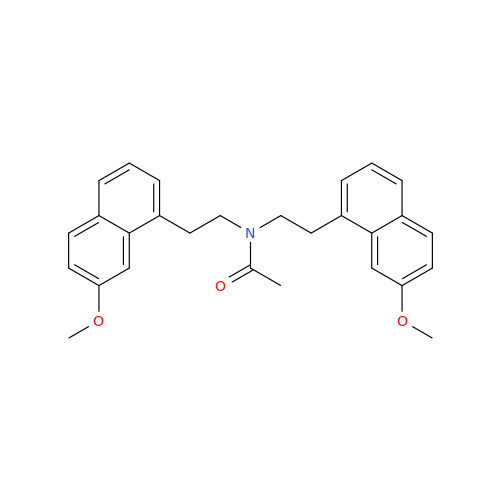
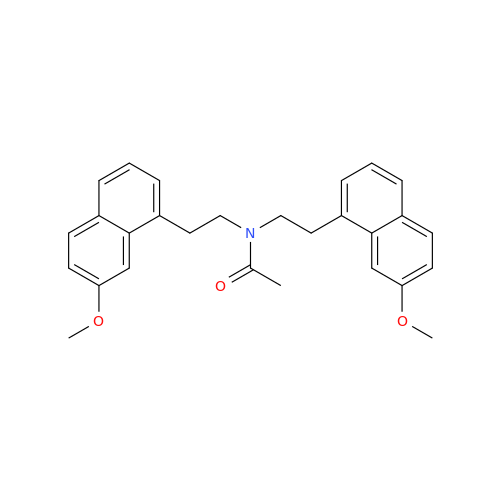
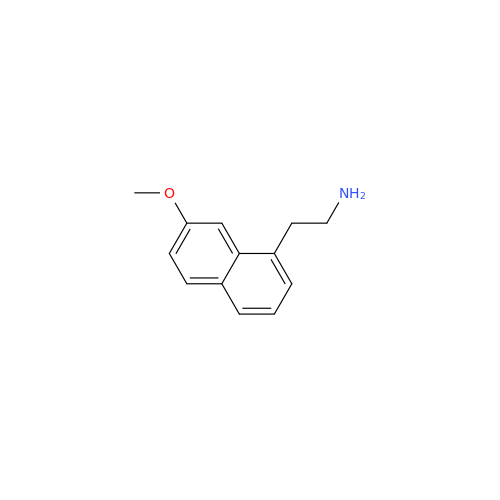
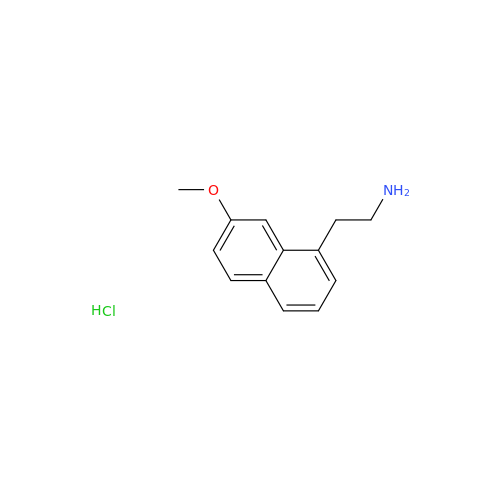
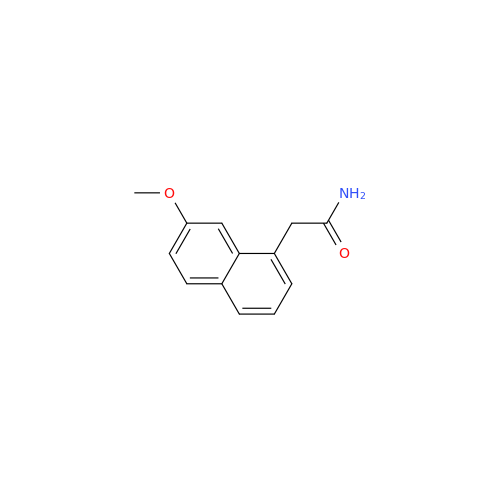
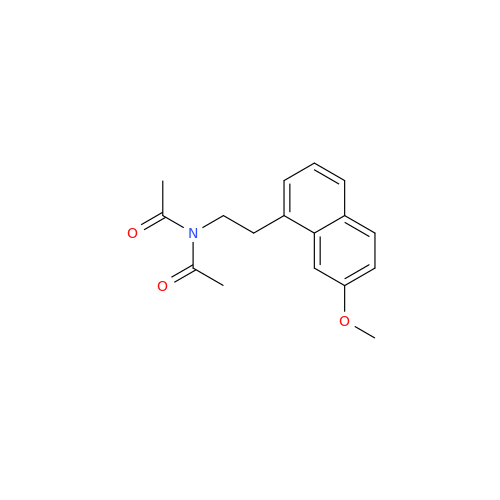
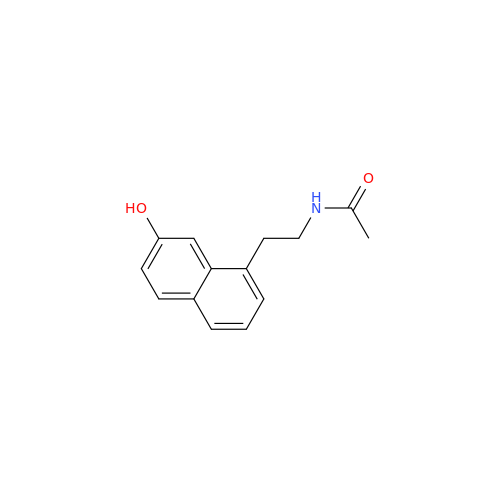
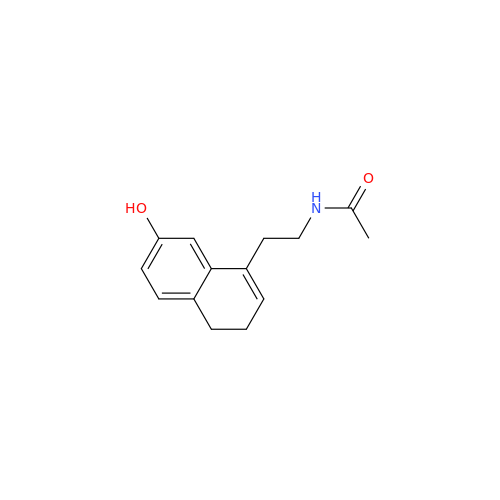
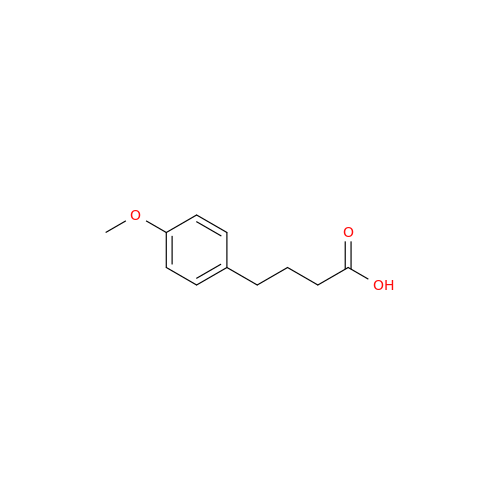
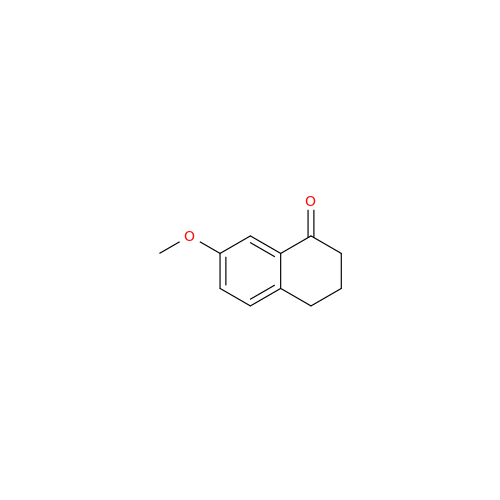
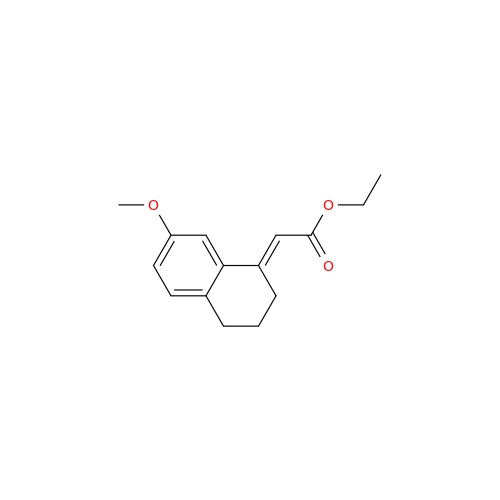
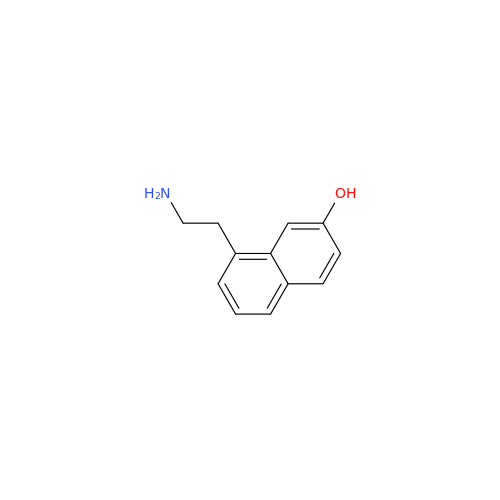
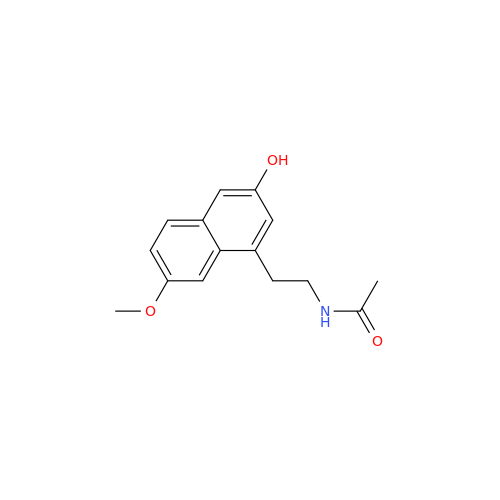
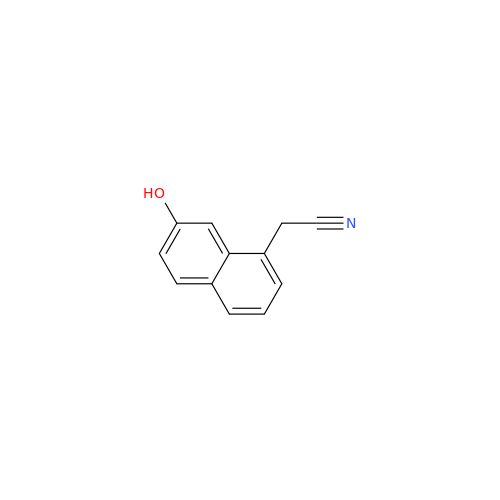
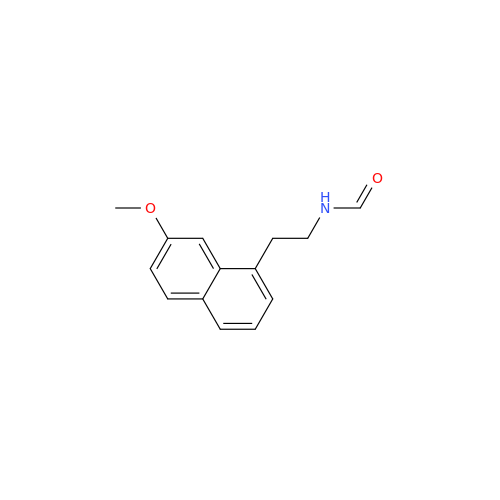
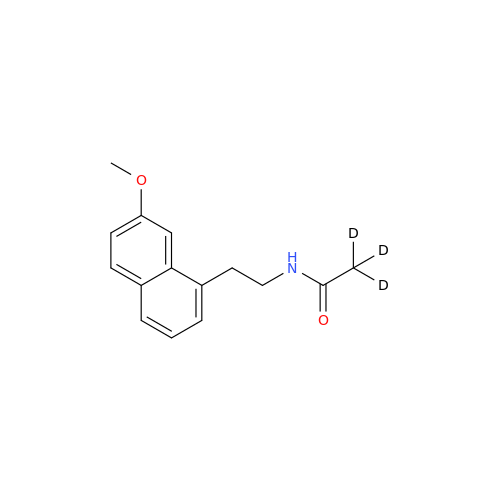
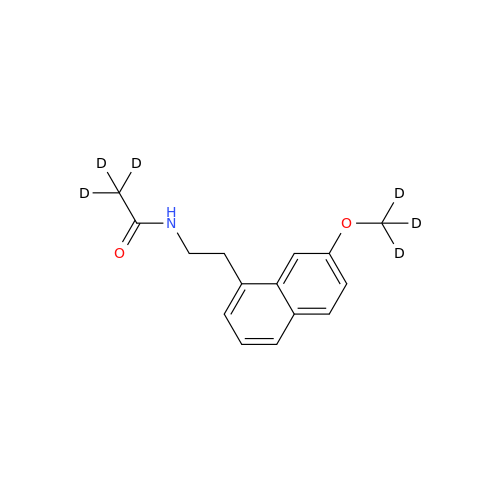
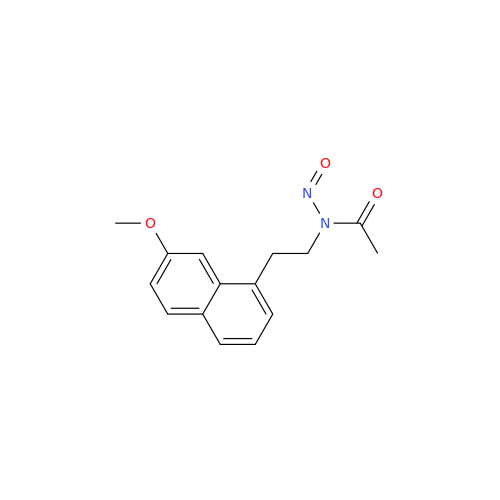
Agomelatine Impurity 9
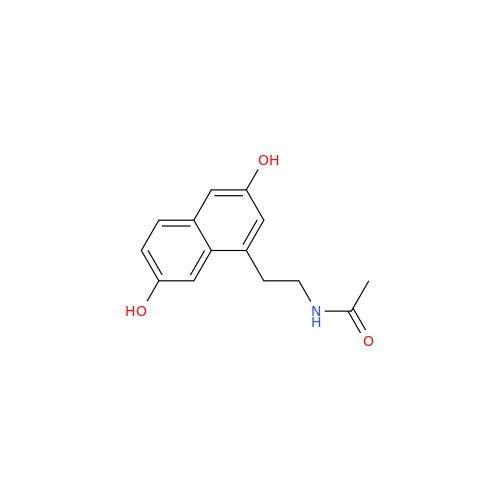
|
Chemical Name: Agomelatine Impurity 9
Synonym: N-[2-(3,7-Dihydroxy-1-naphthalenyl)ethyl]acetamide| Enter Batch Number | |||