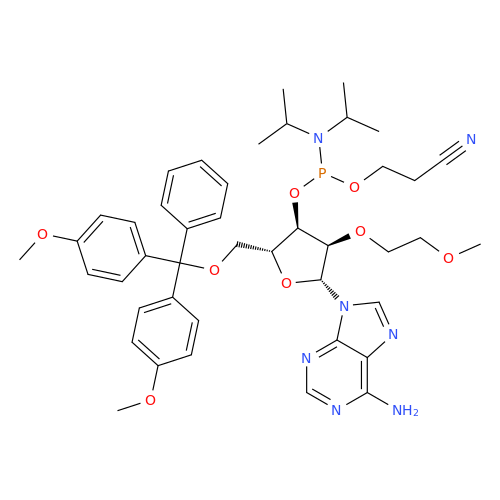
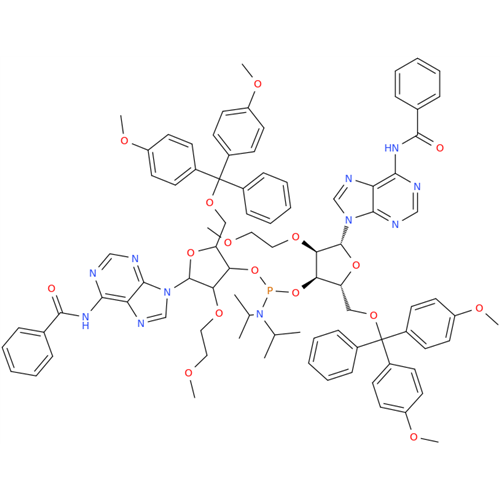
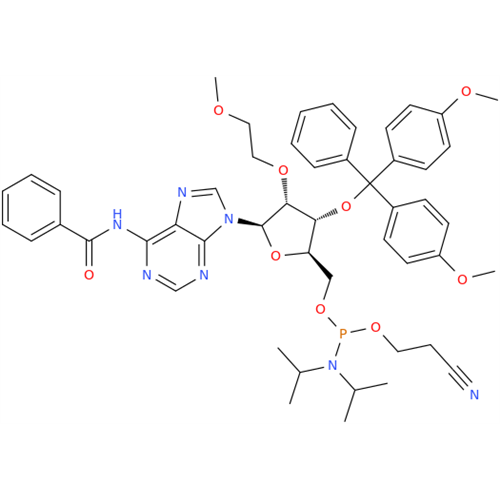
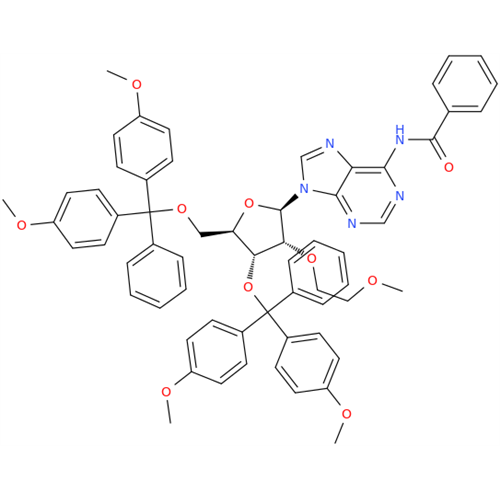
Product Information
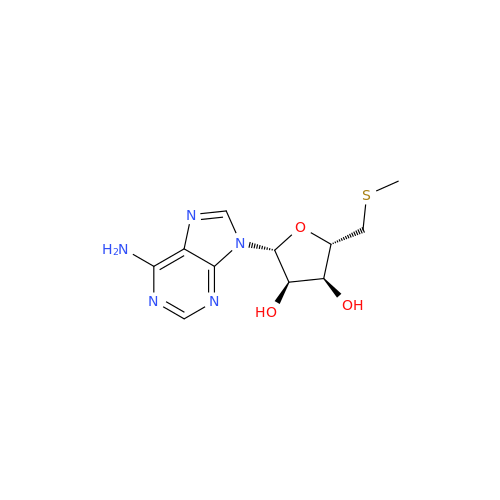
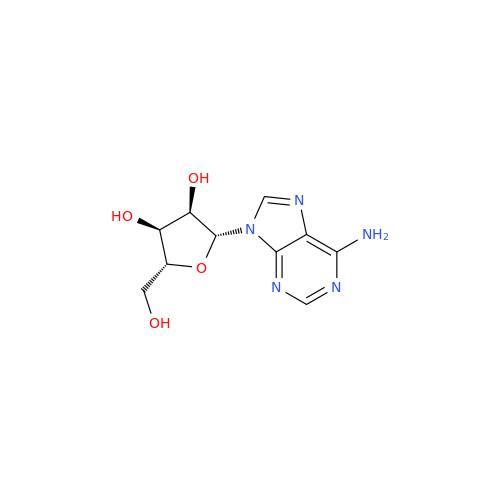
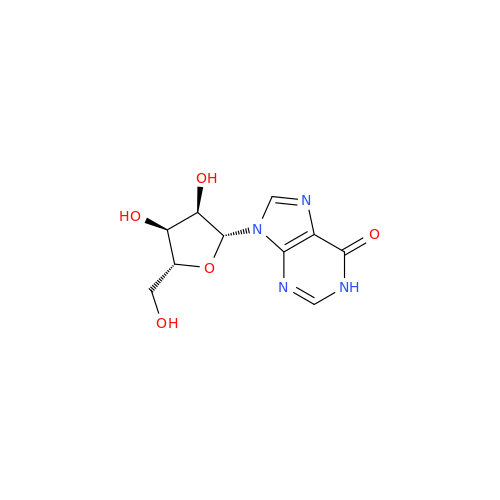
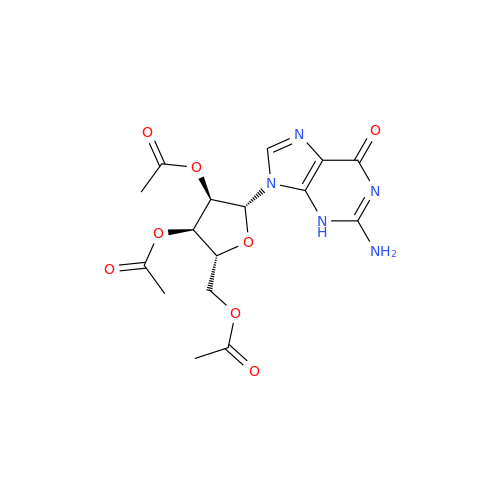
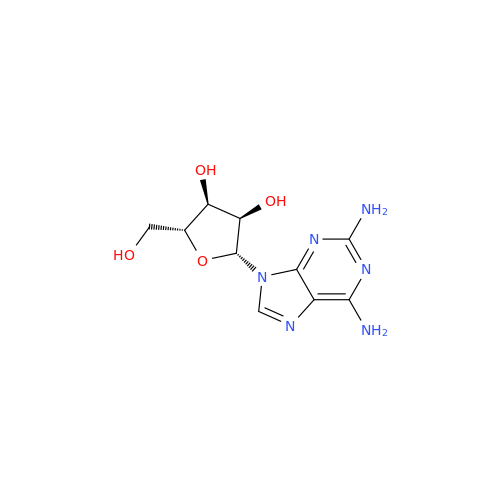
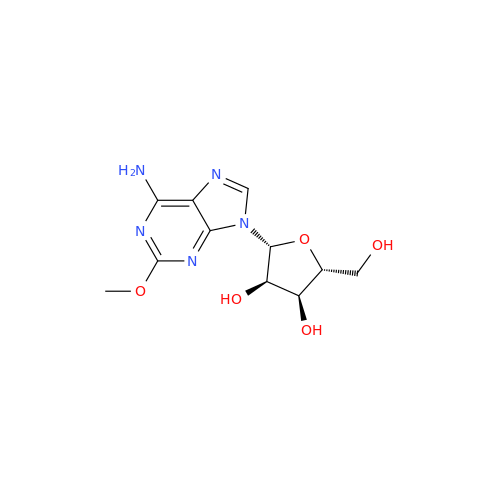
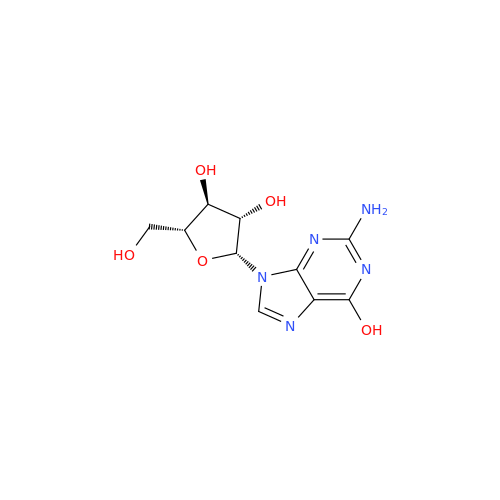
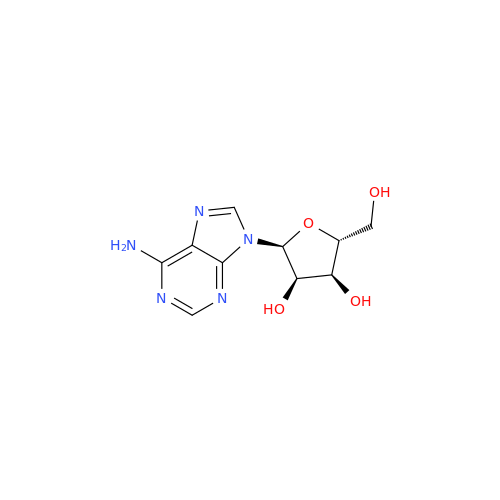
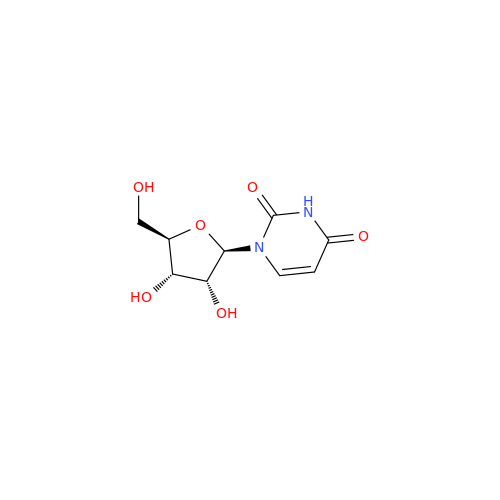
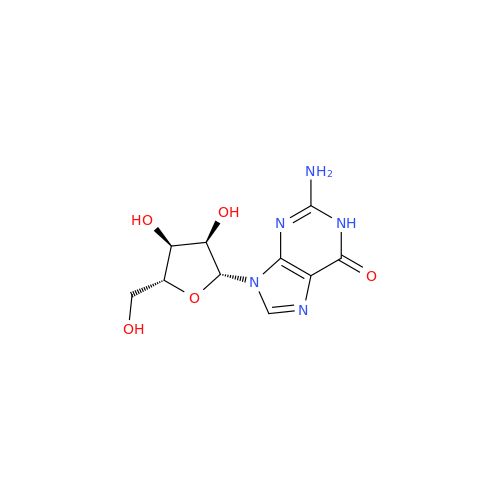
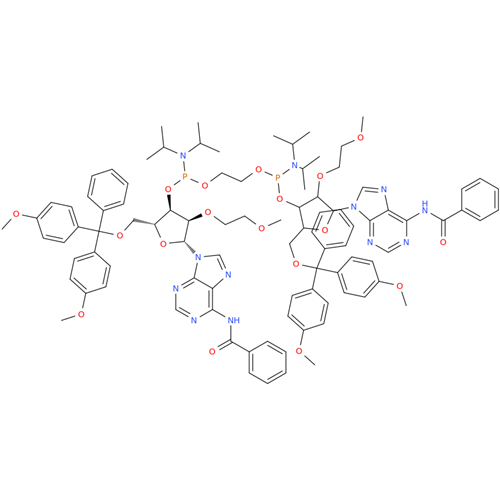
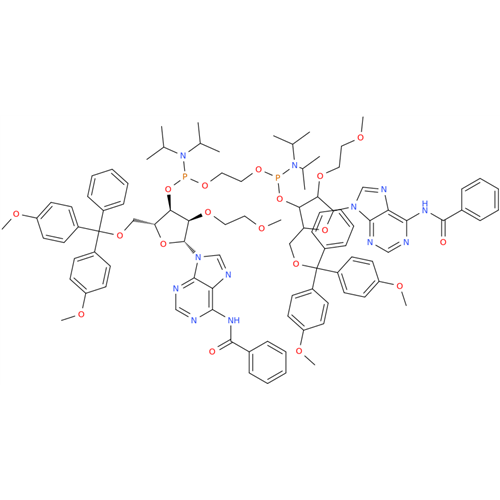
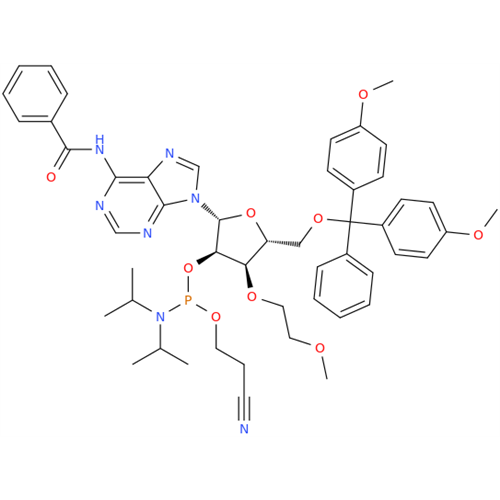
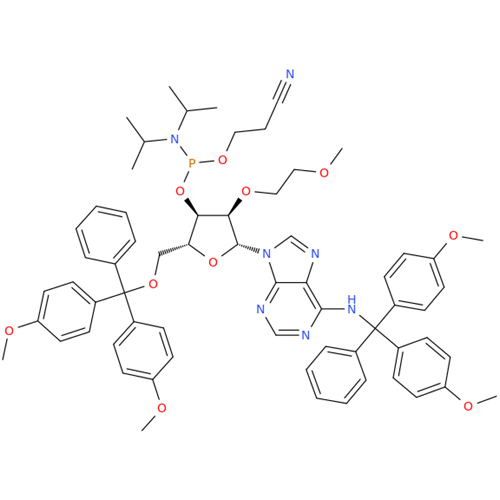
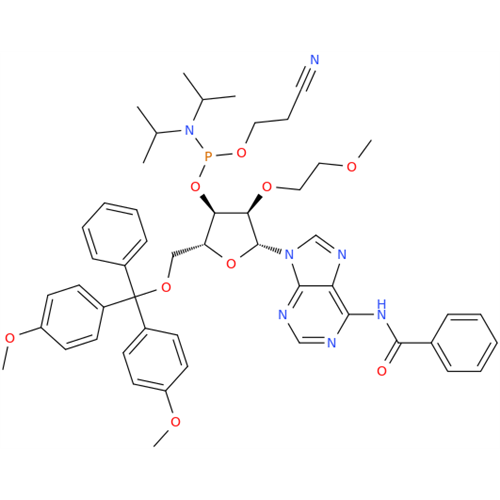
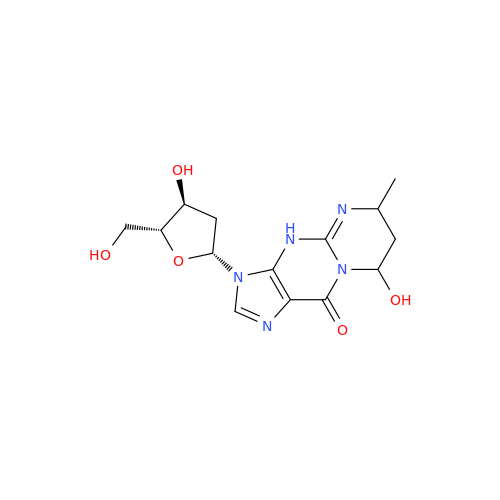
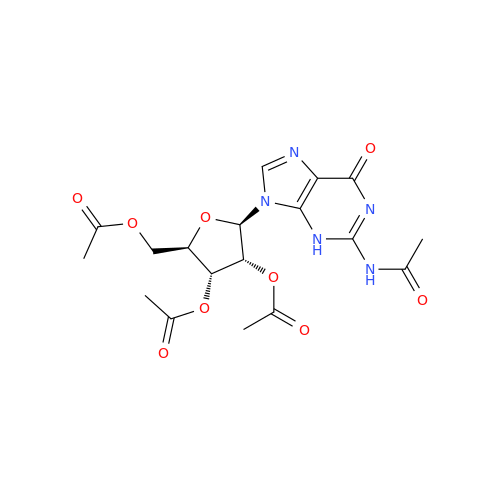
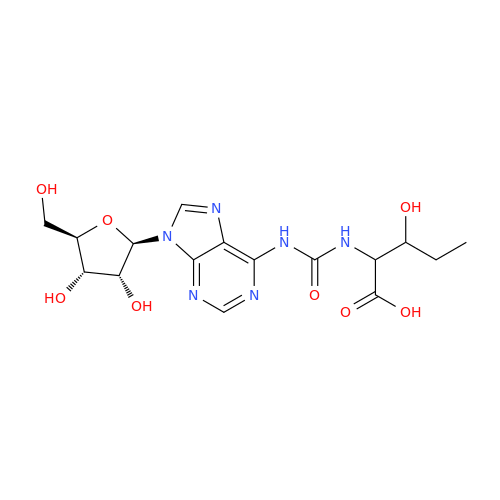
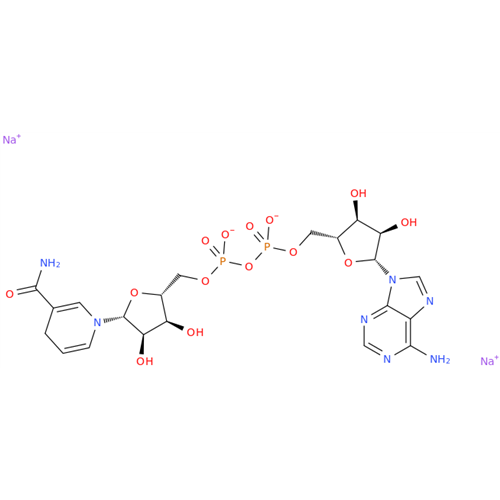
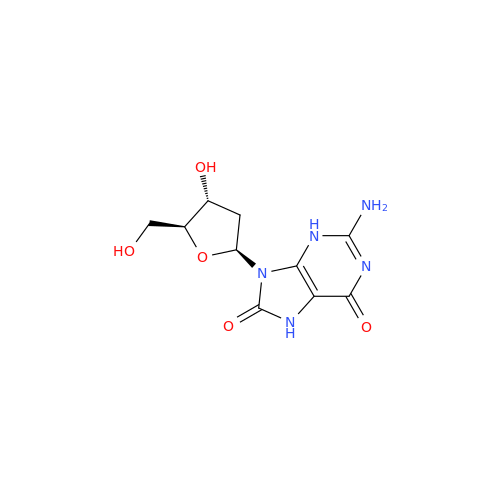
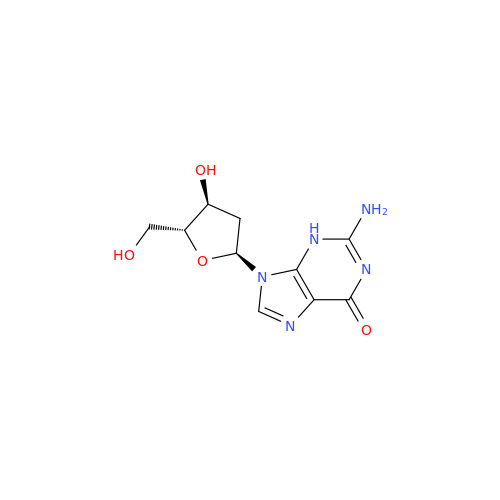
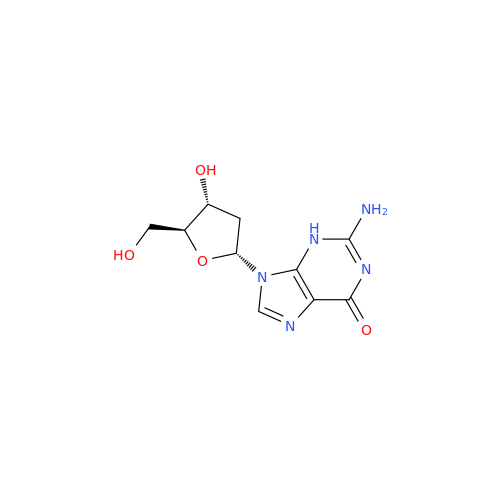
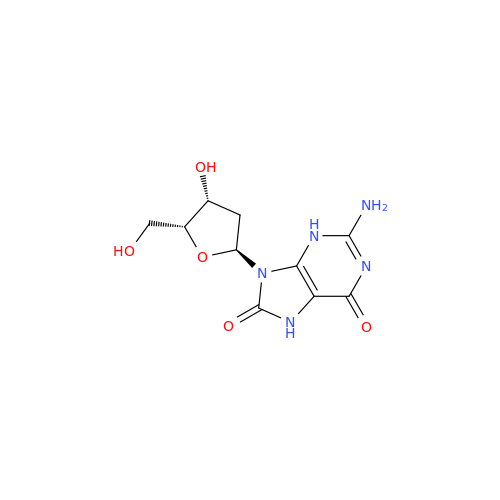
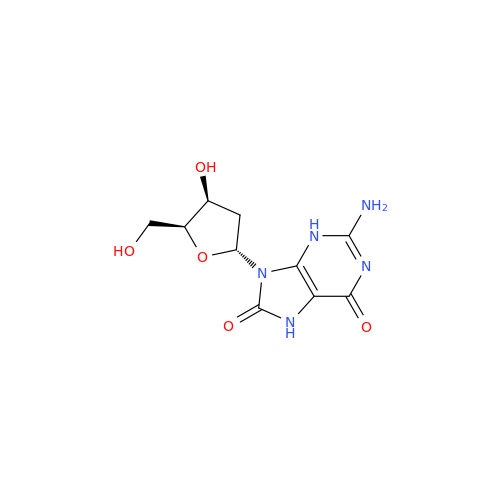
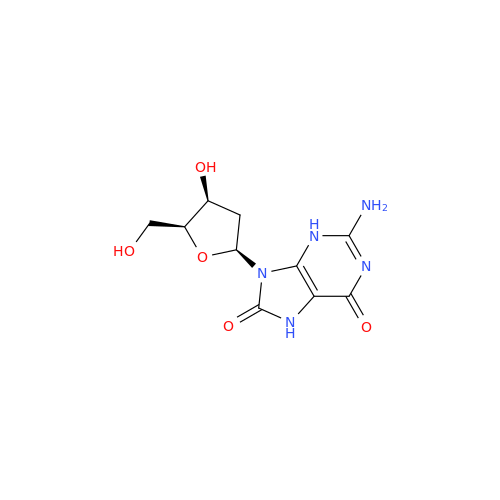
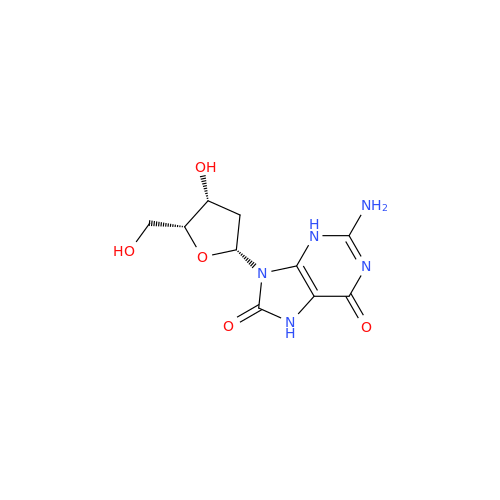
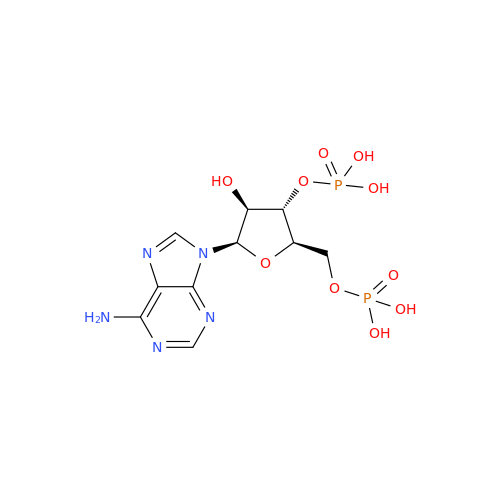
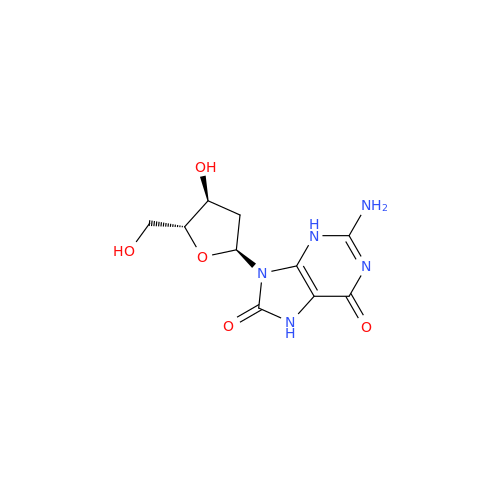
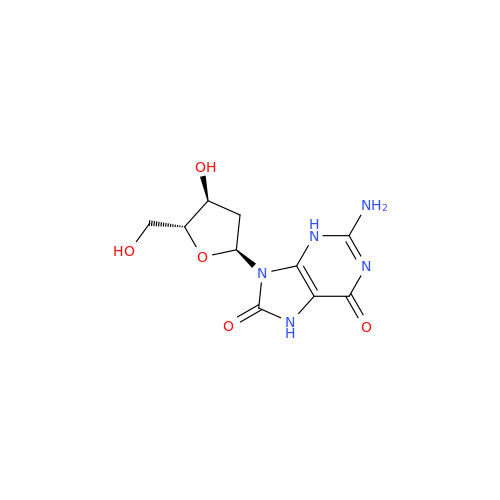
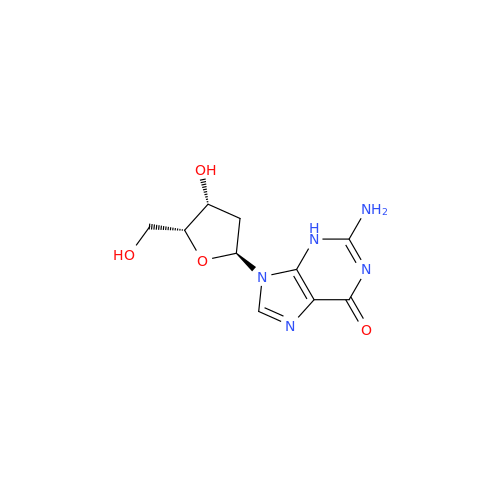
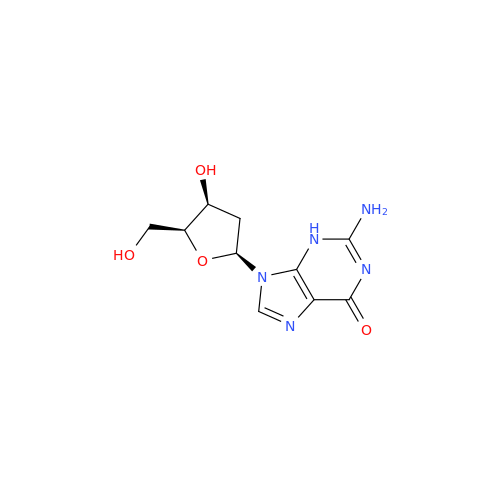
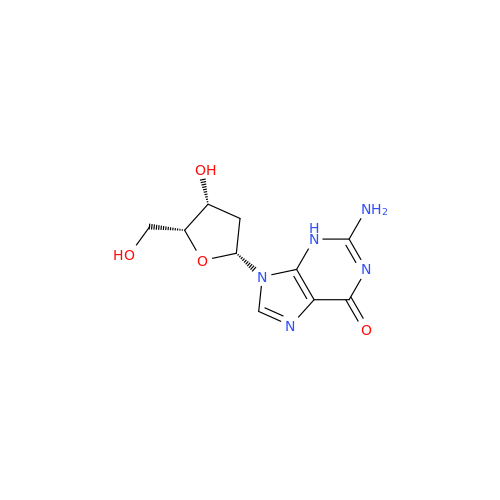
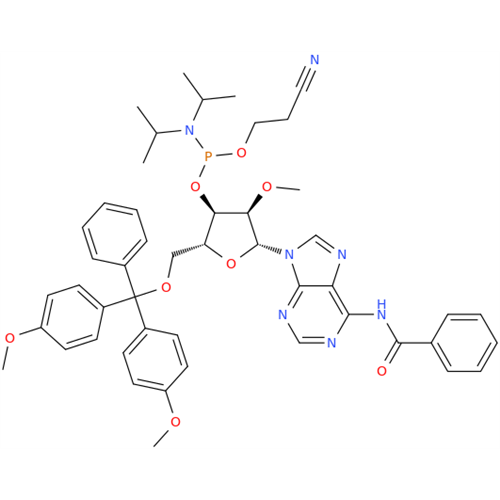
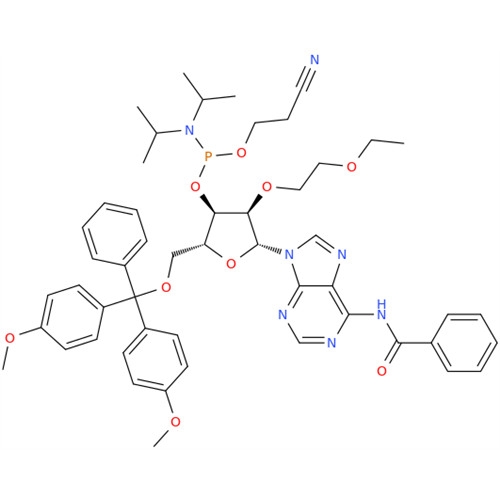
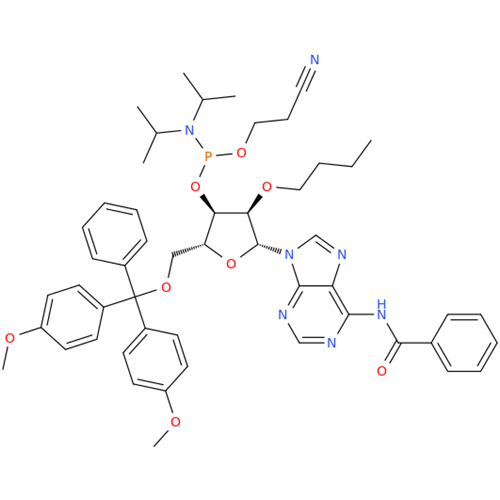
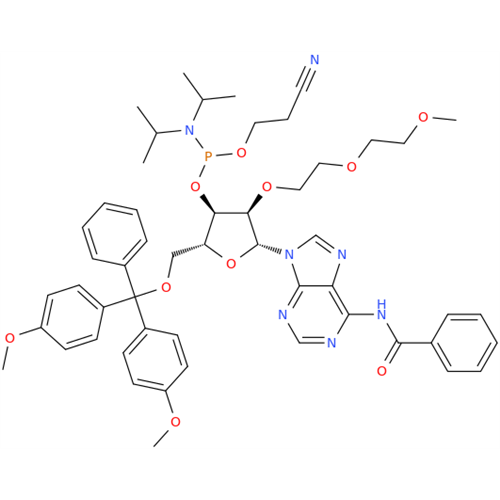
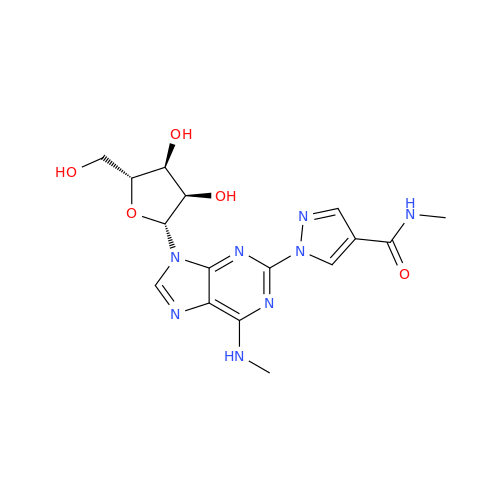
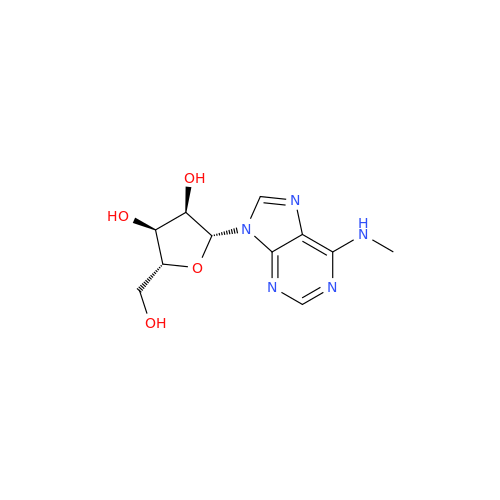
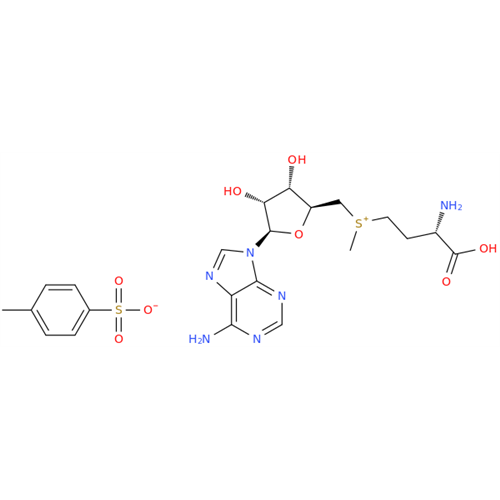
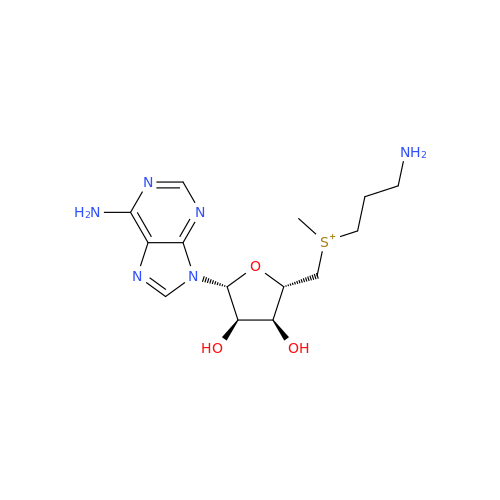
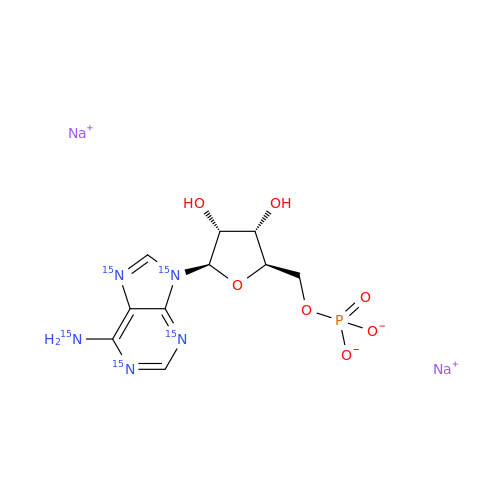
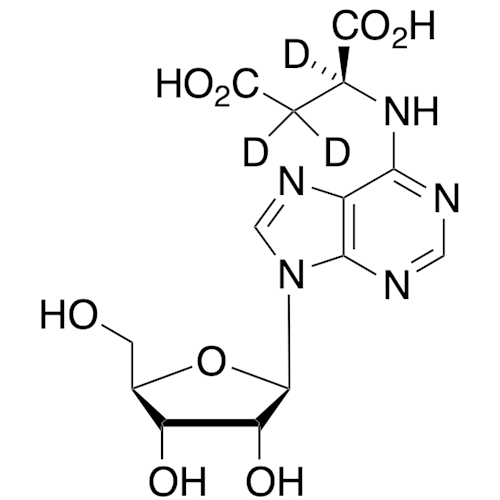
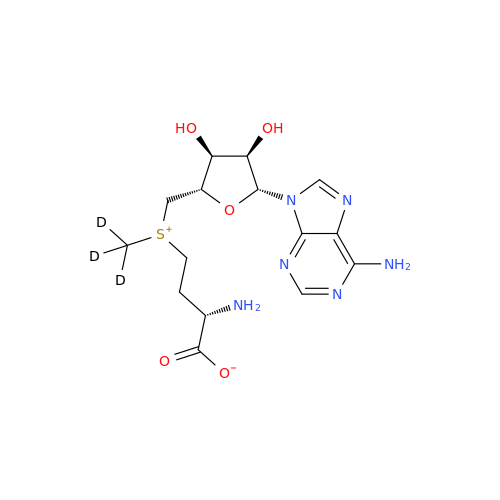
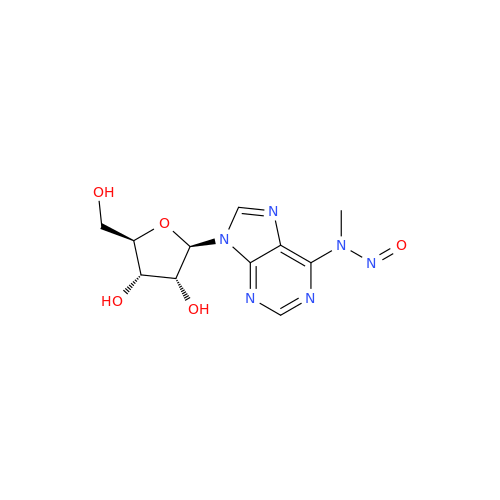
Adenosine Impurity 33
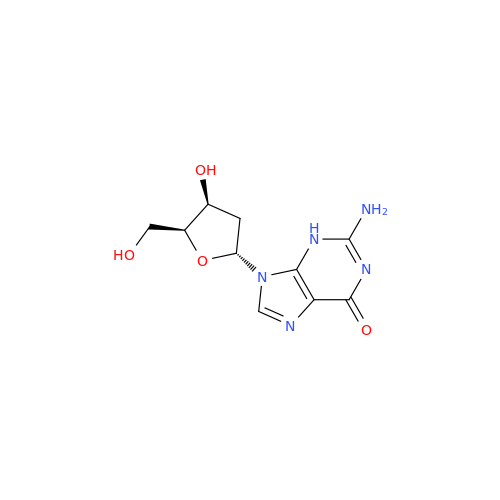
|
Chemical Name: Adenosine Impurity 33
Synonym: 2-Amino-9-((2R,4S,5S)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-3,9-dihydro-6H-purin-6-one| Enter Batch Number | |||