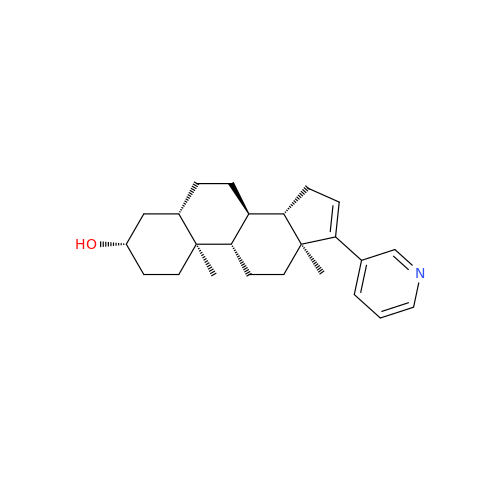
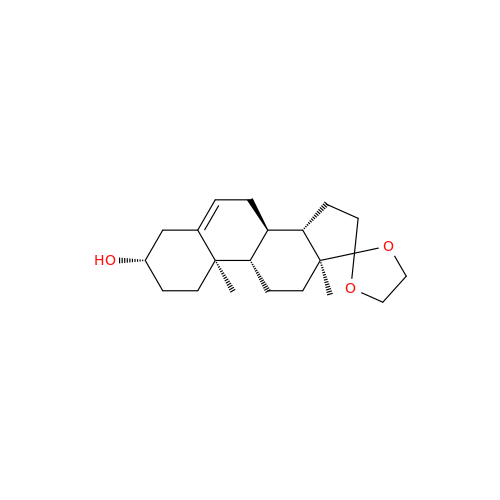
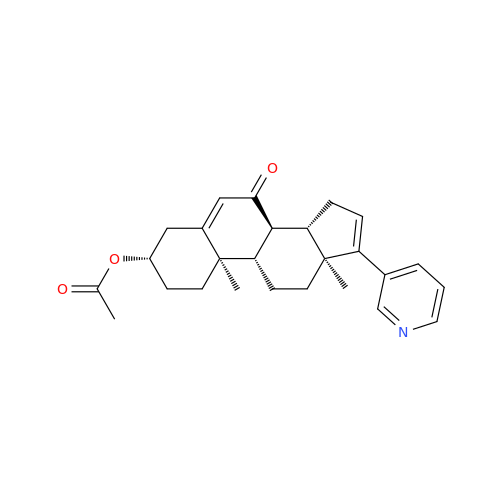
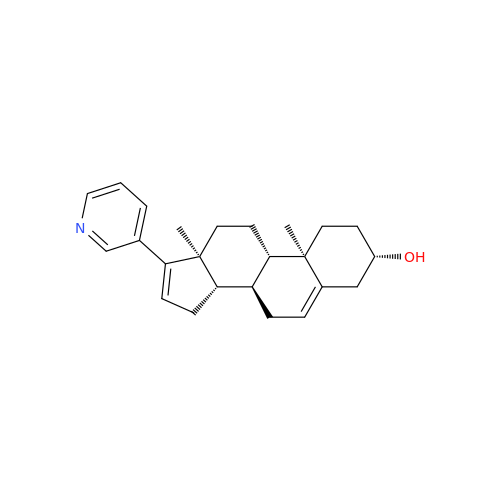
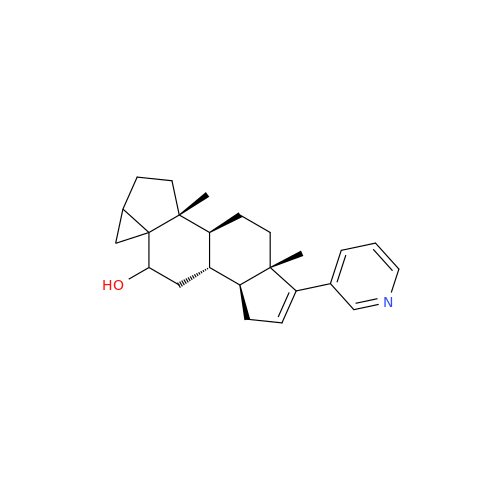
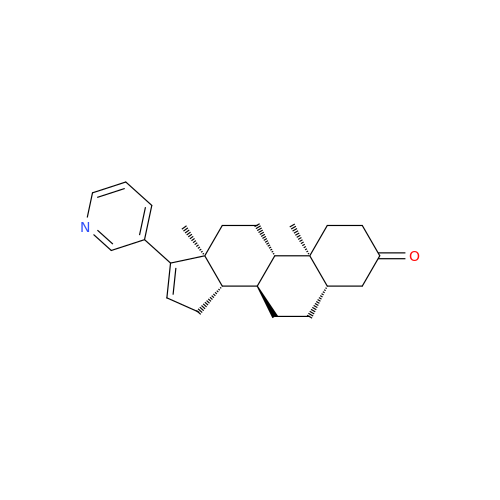
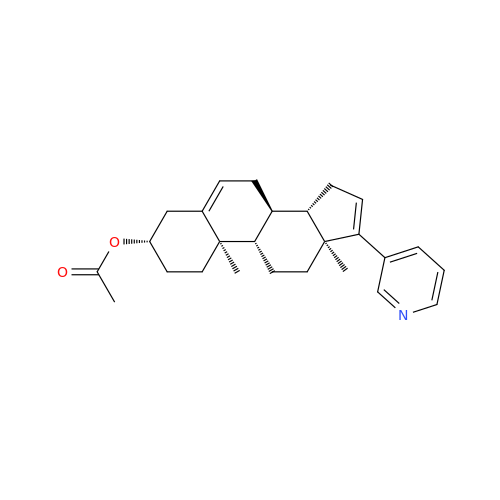
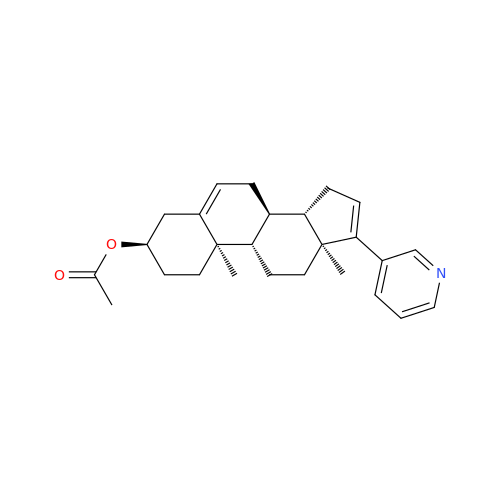
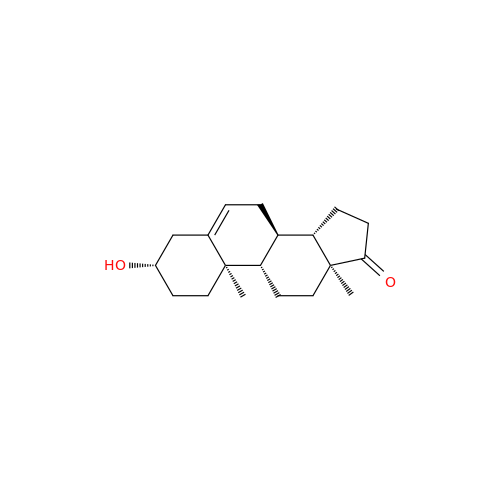
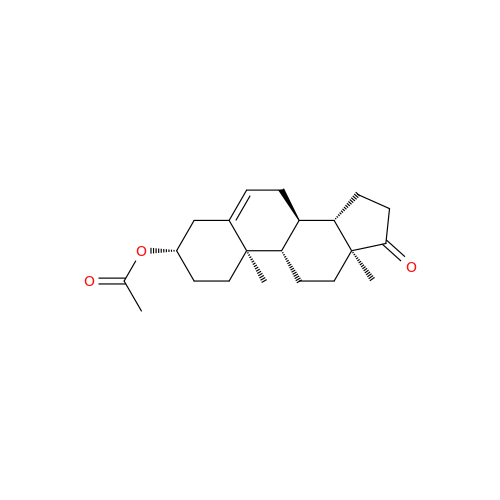
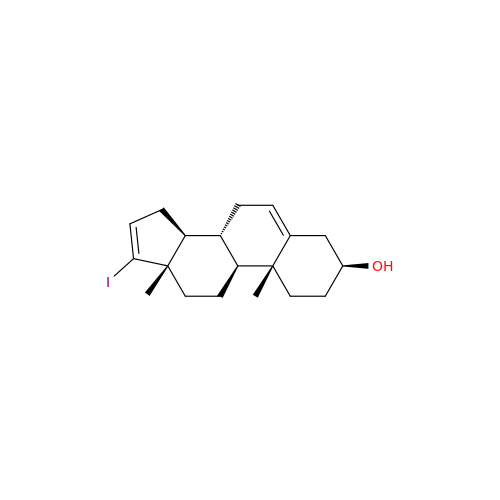
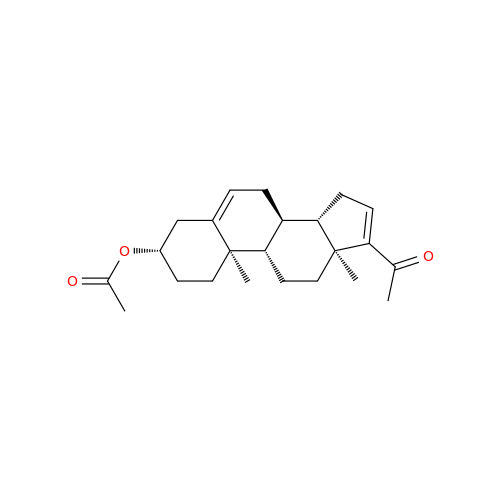
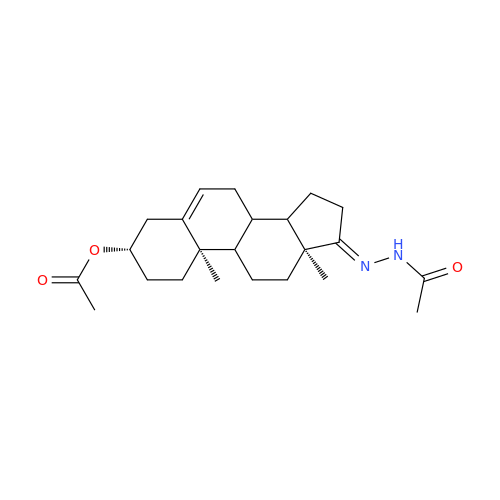
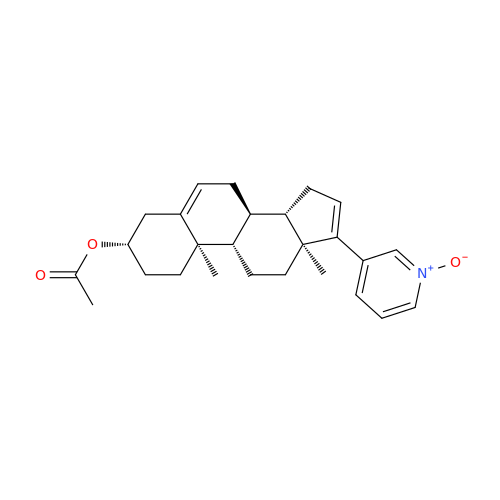
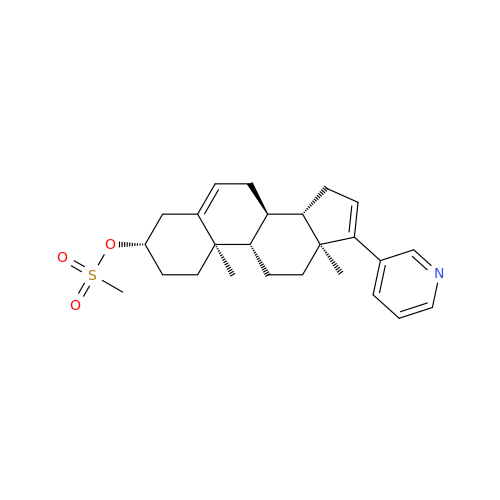
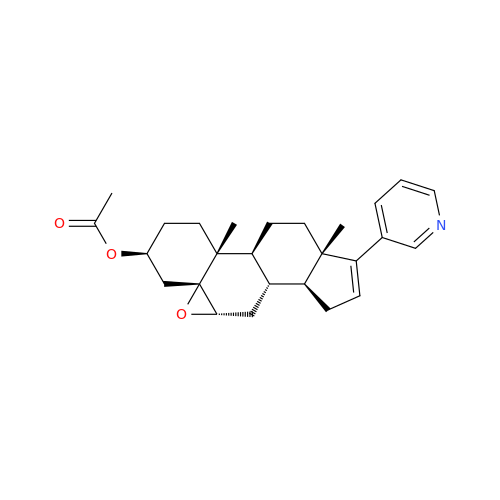
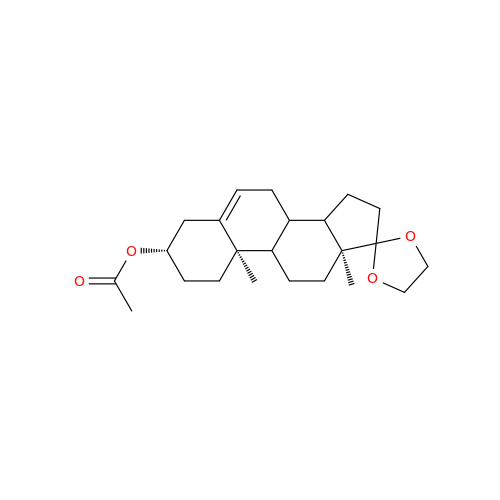
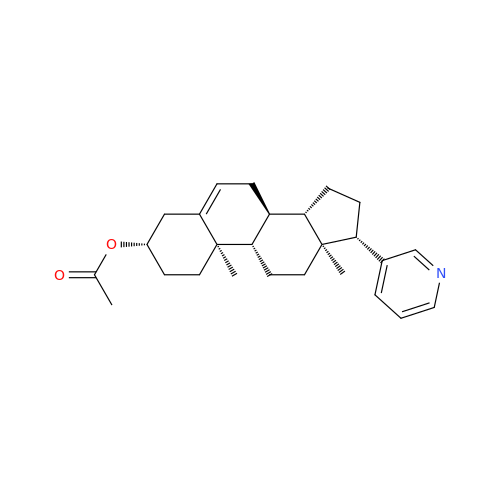
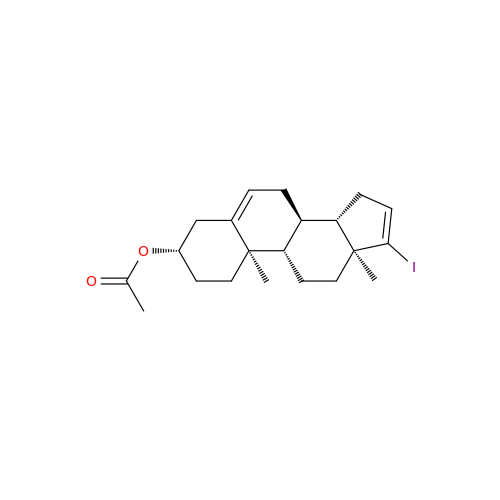
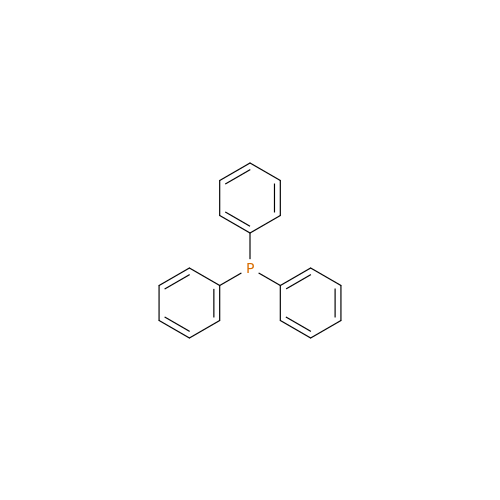
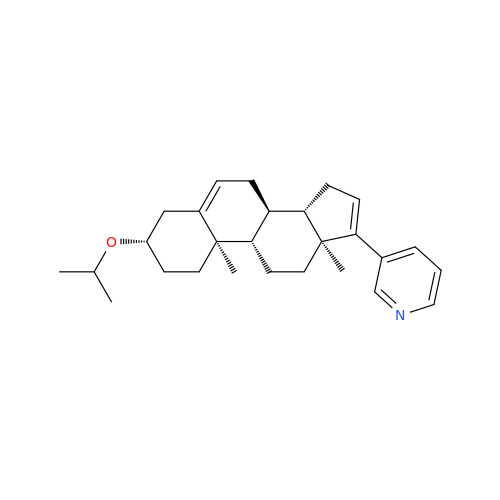
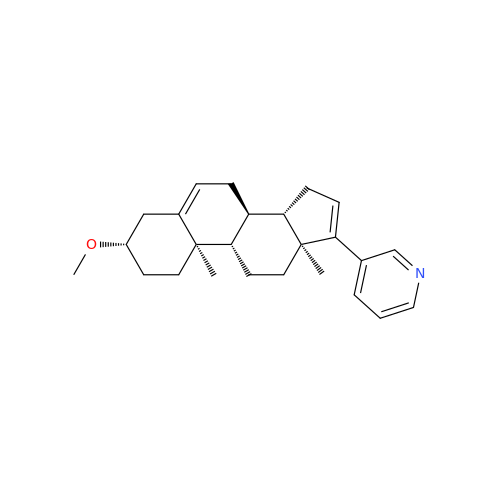
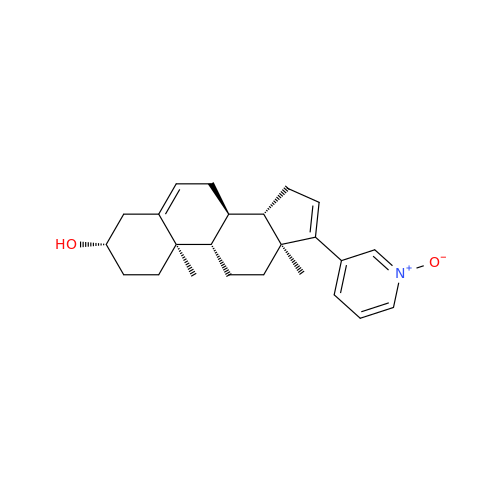
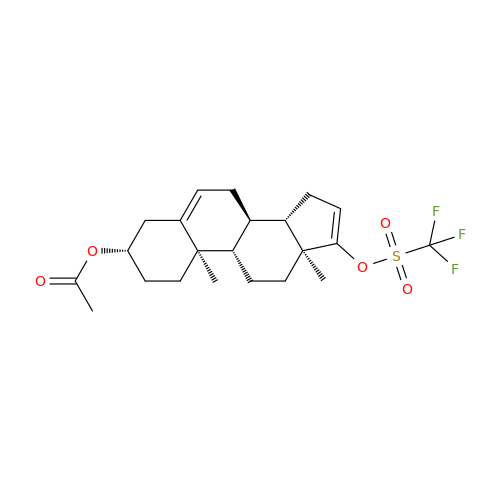
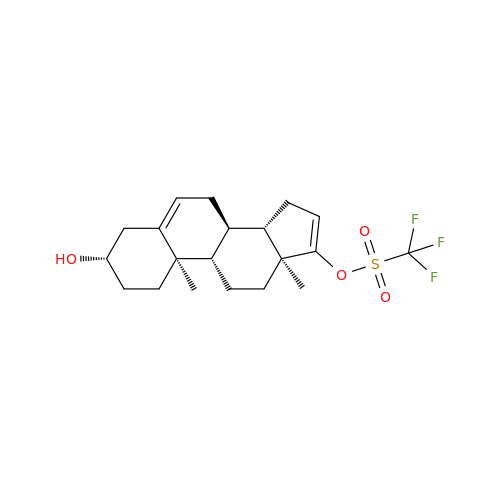
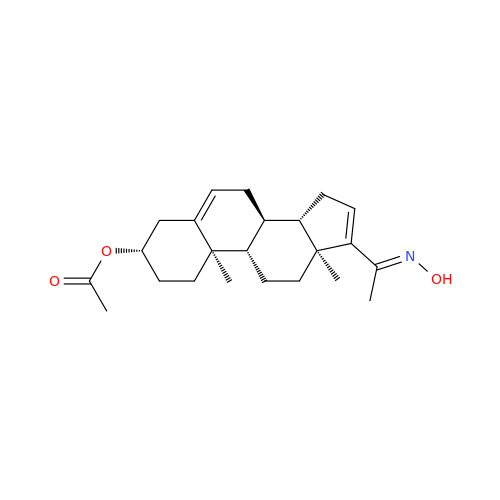
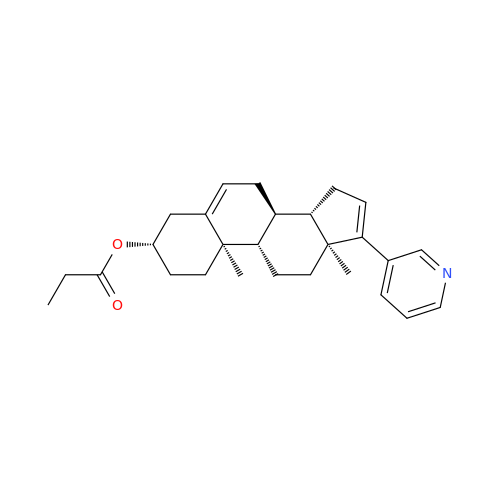
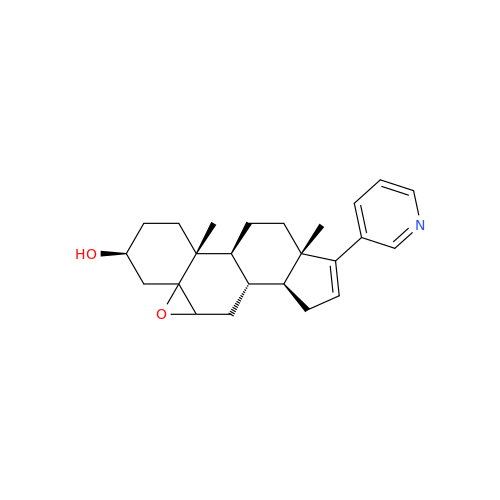
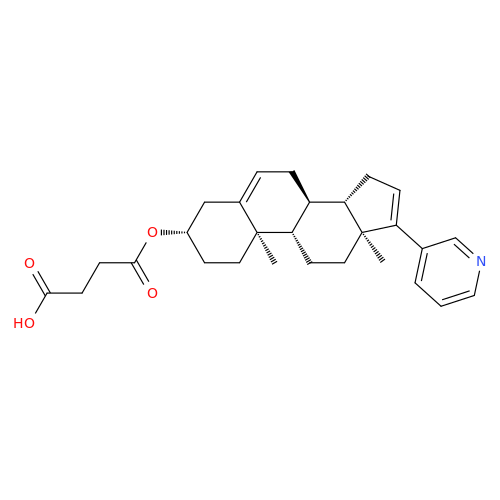
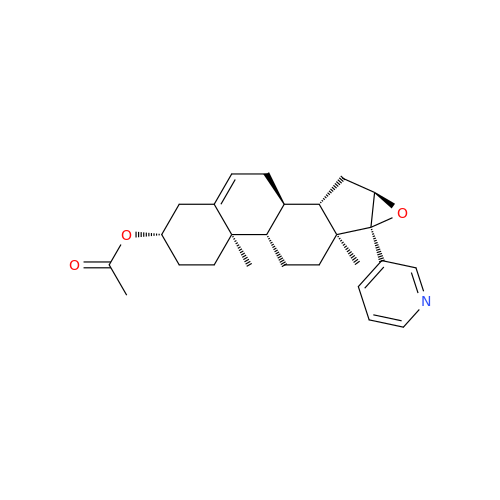
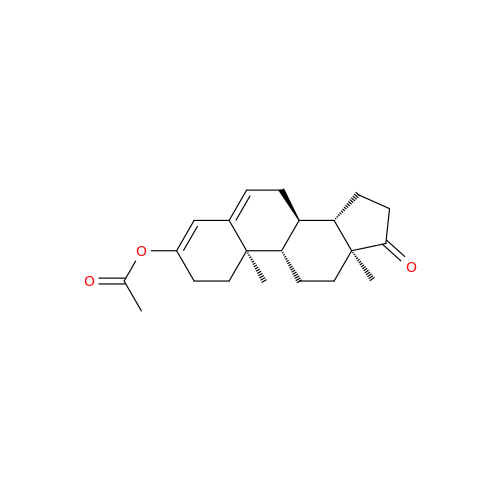
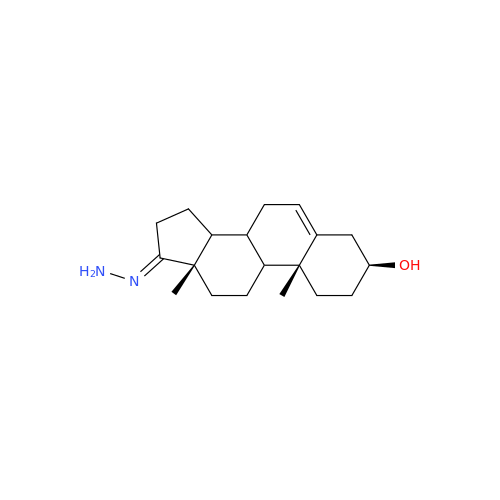
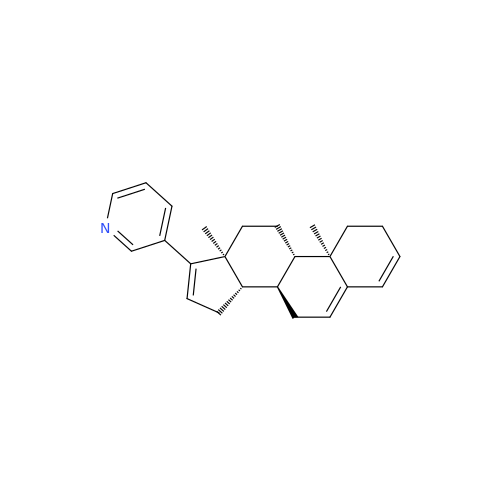
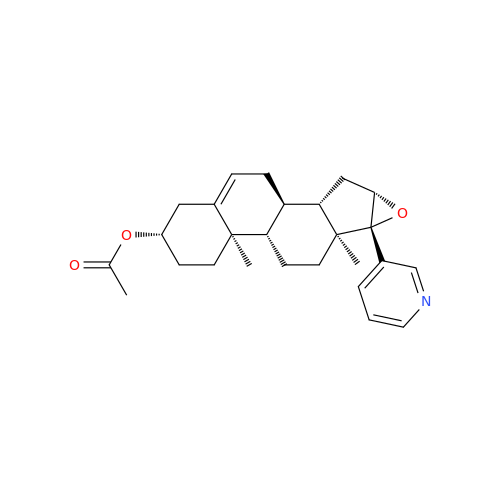
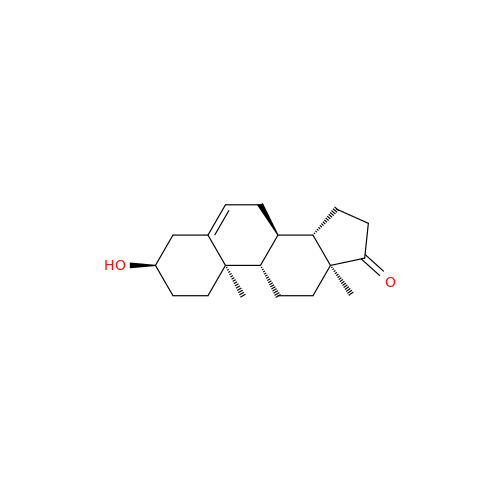
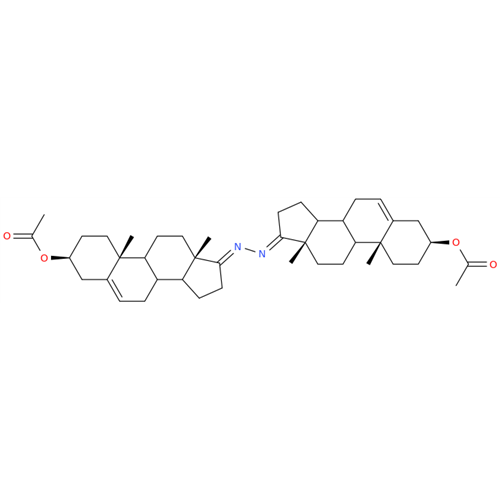
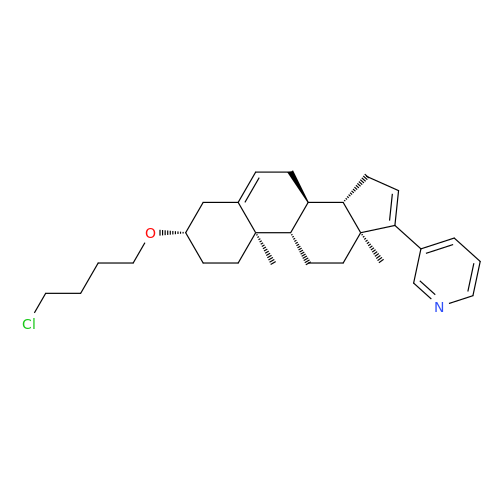
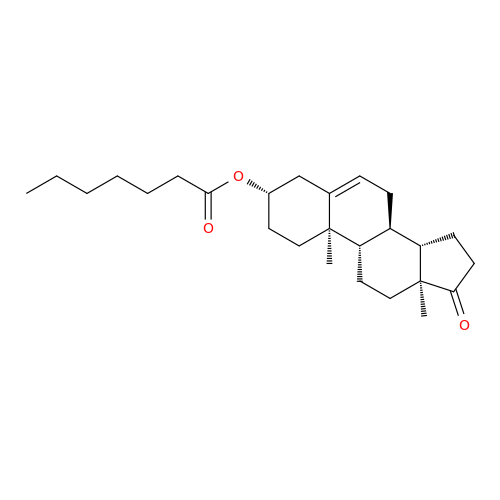
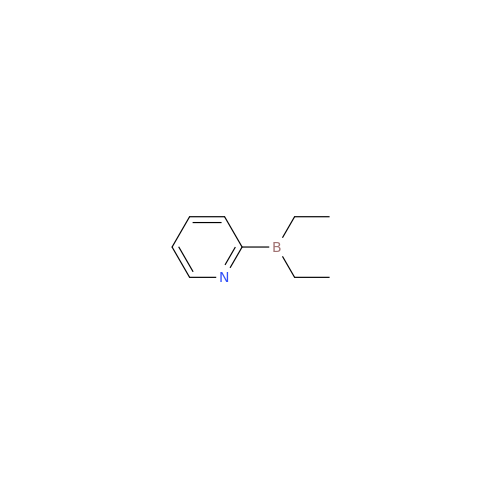
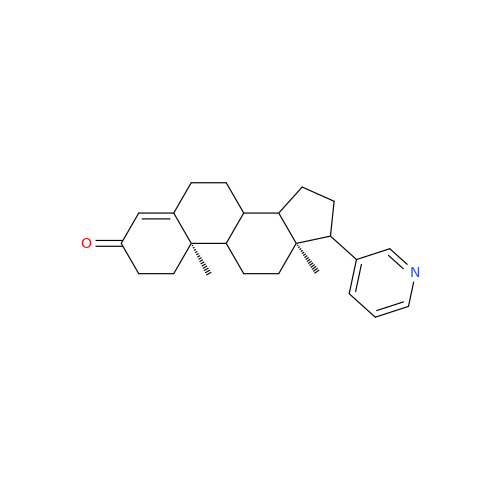
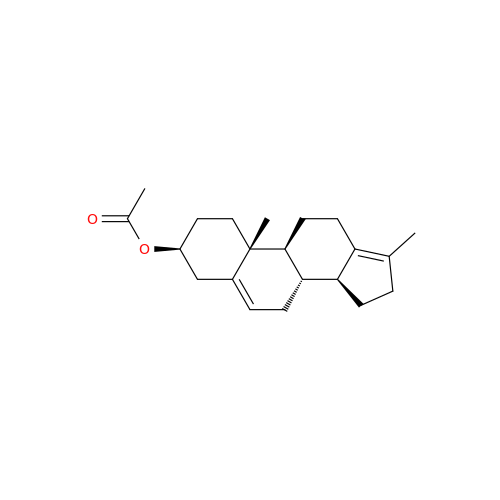
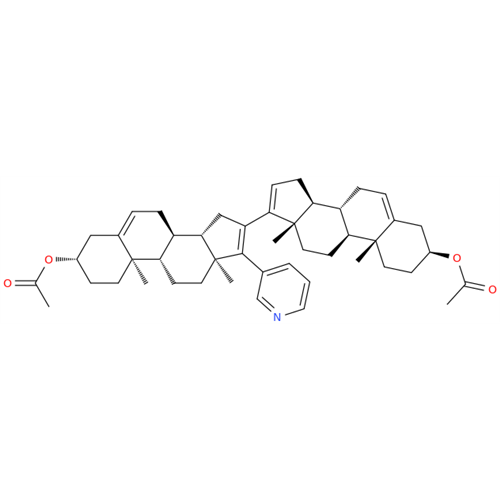
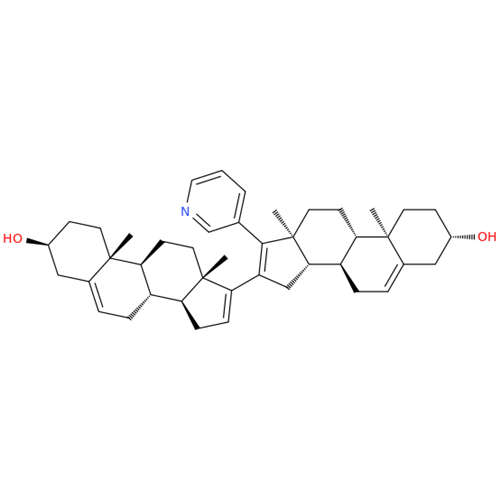
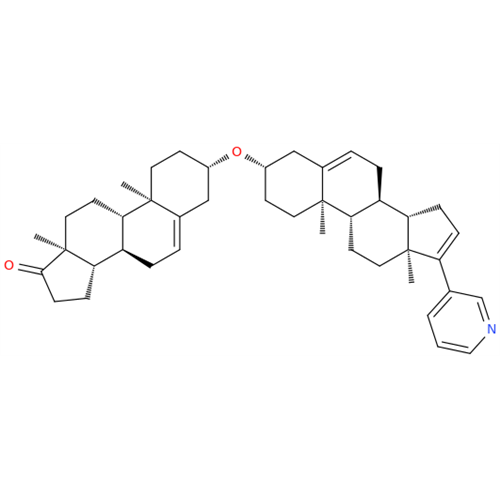
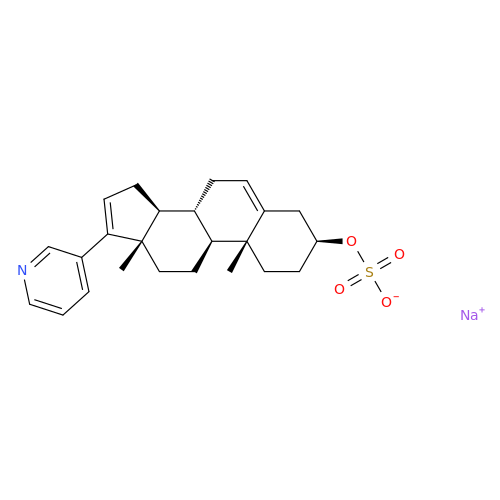
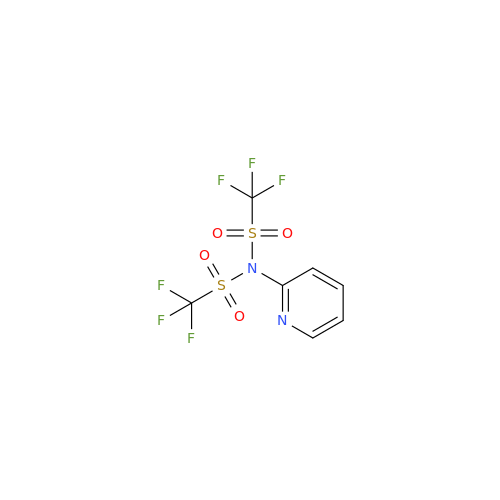
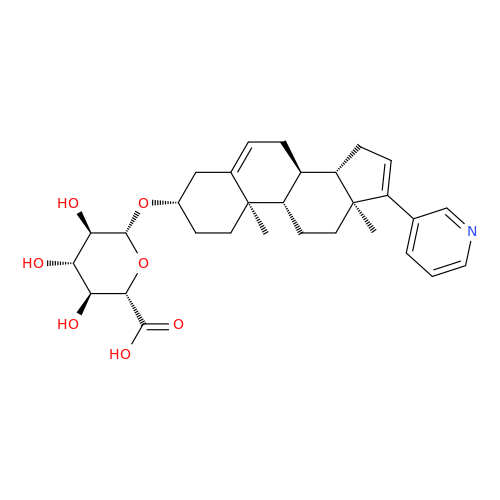
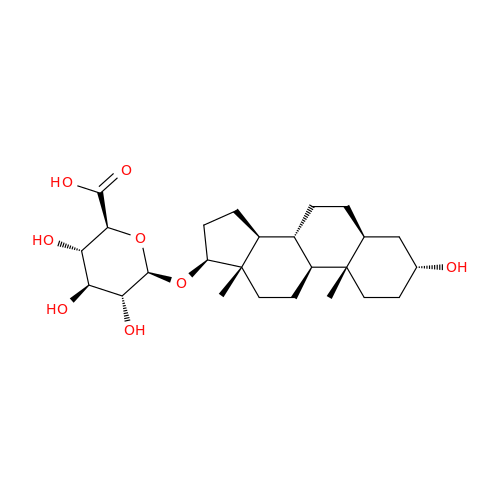
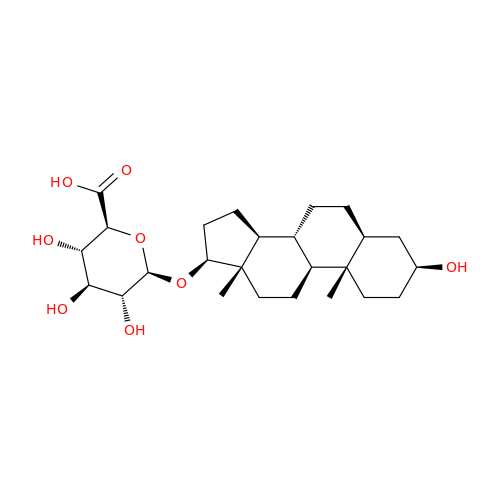
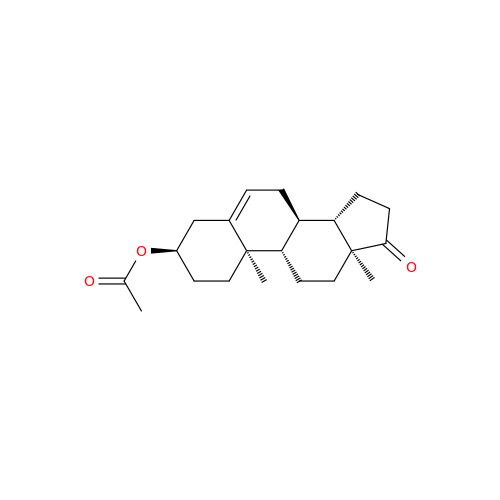
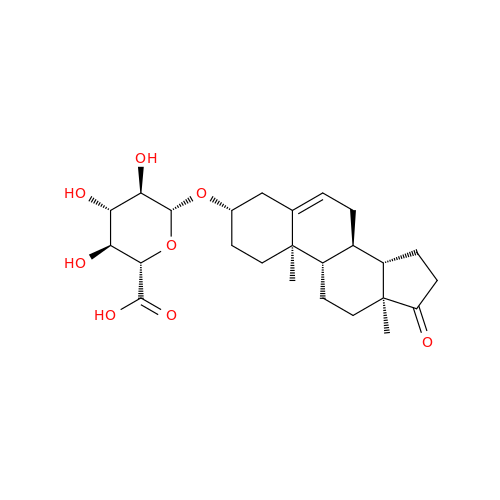
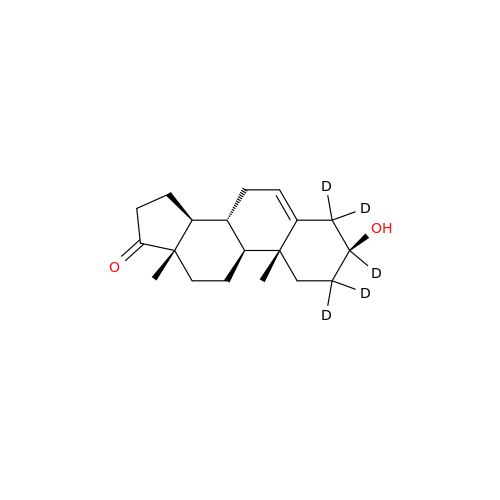
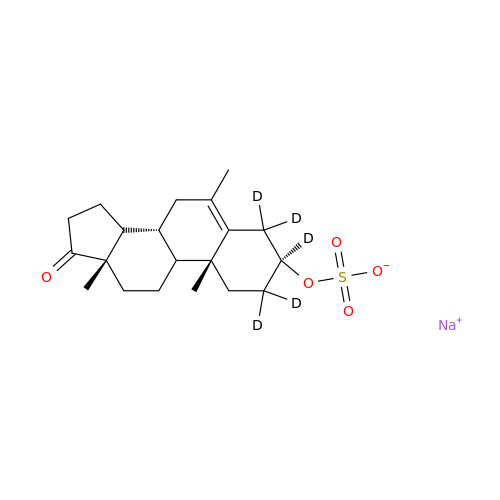
Product Information
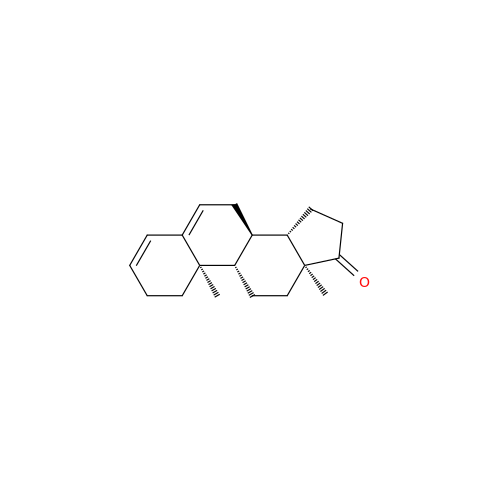
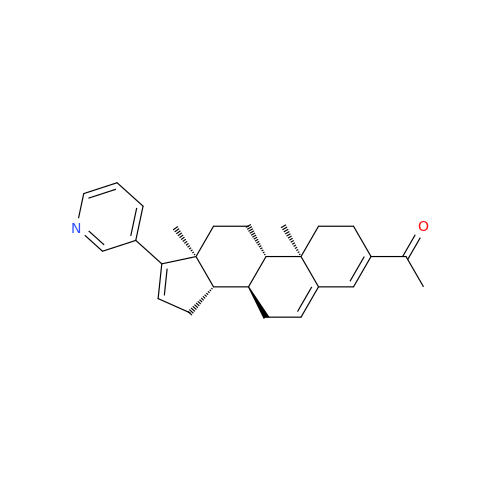
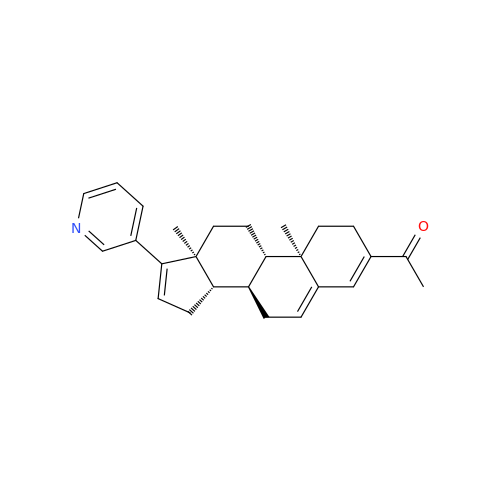
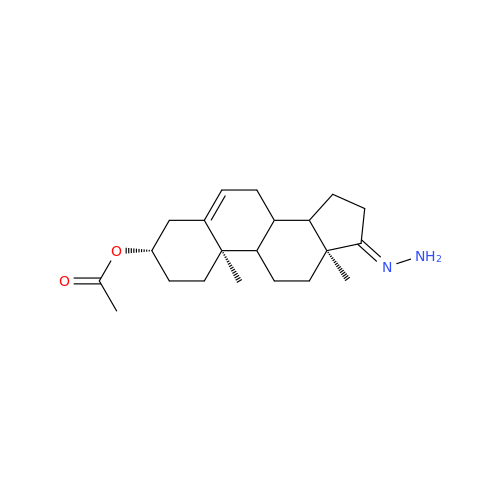
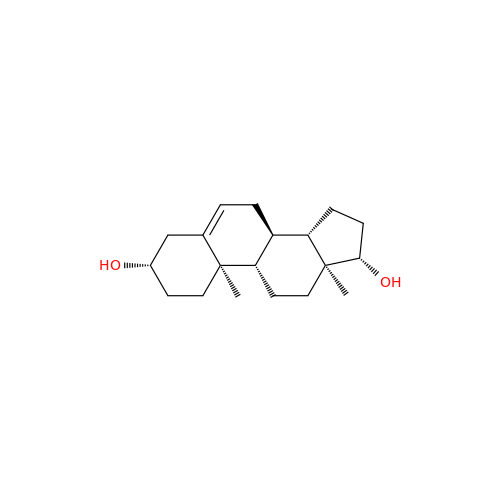
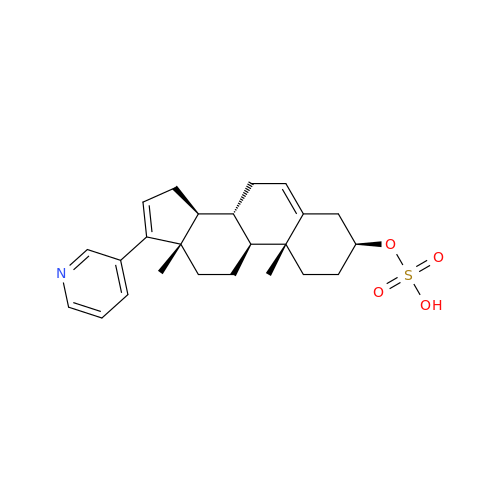
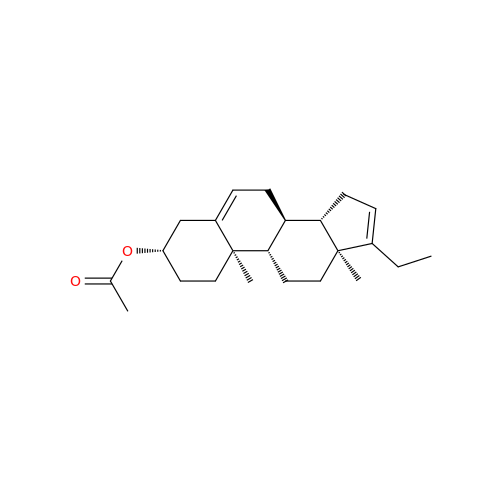
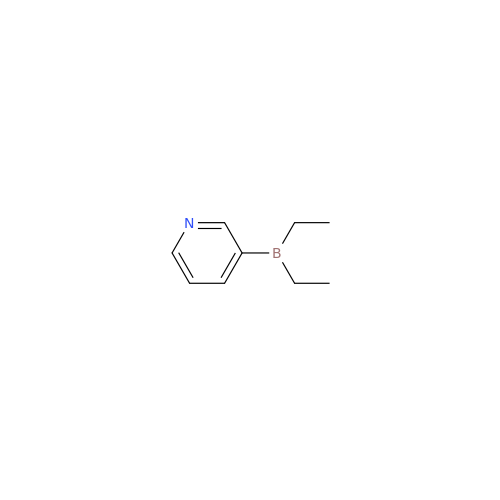
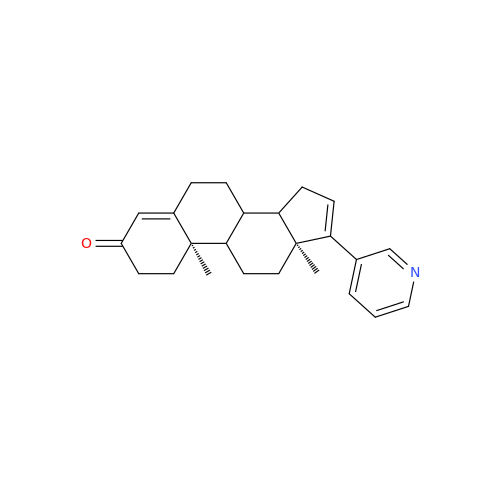
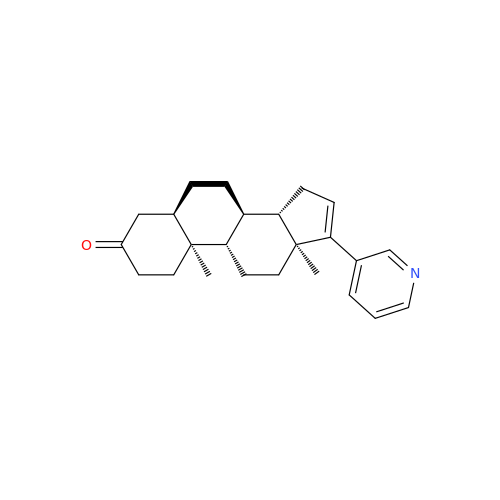
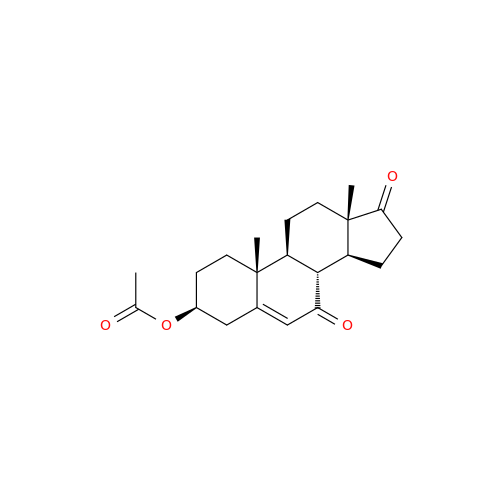
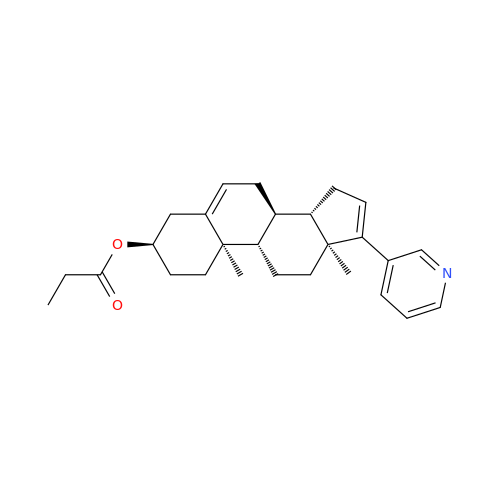
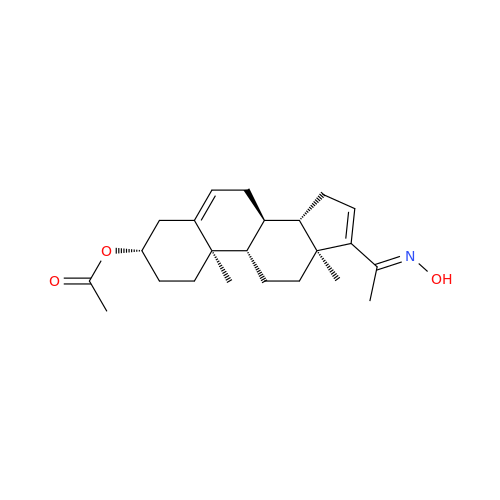
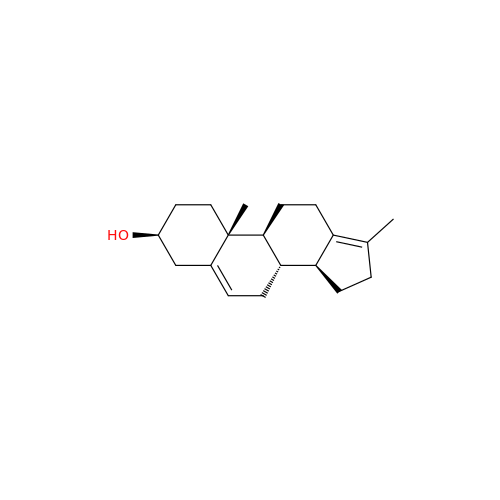
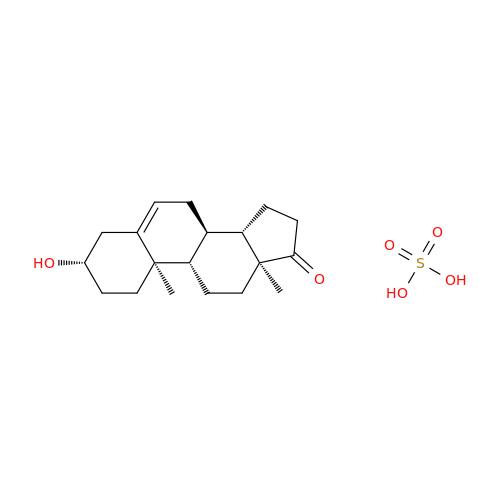
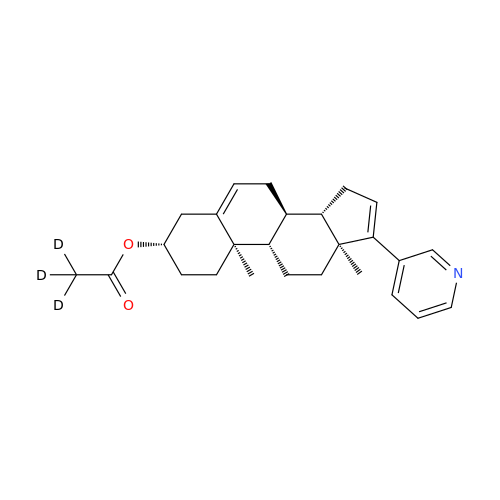
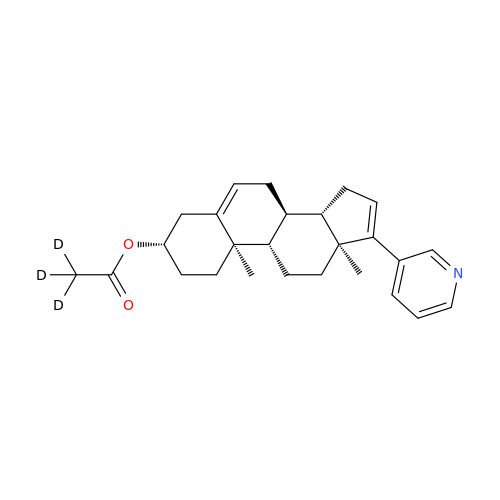
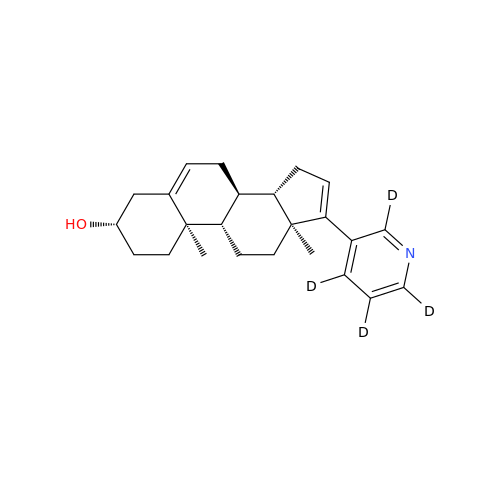
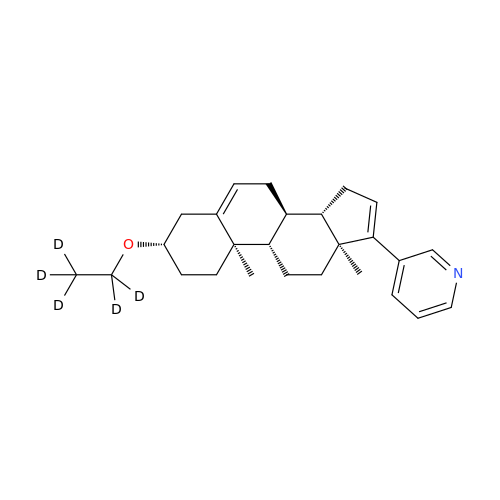
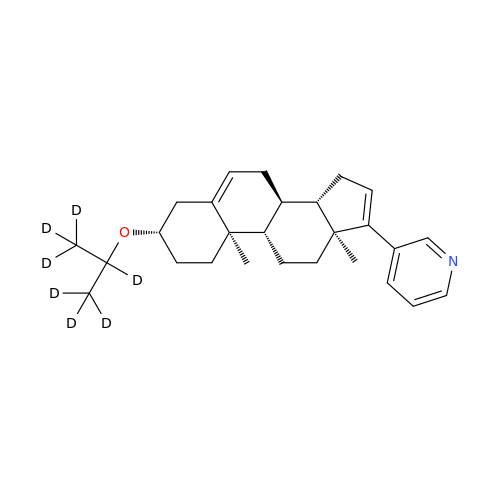
Abiraterone Acetate Reduced Impurity
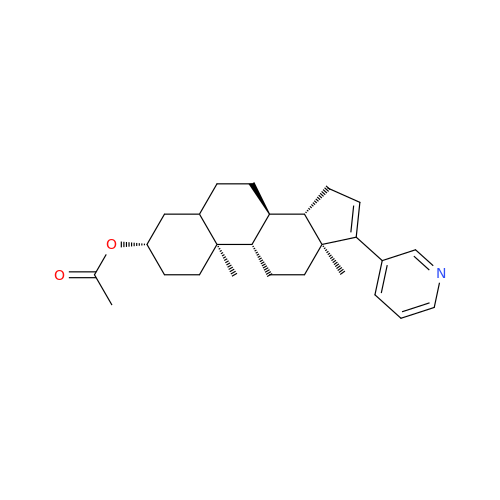
|
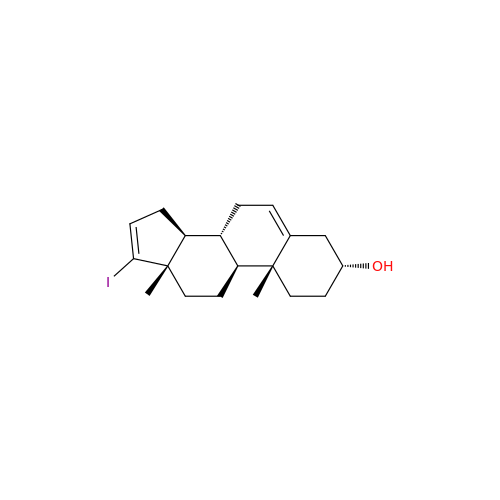
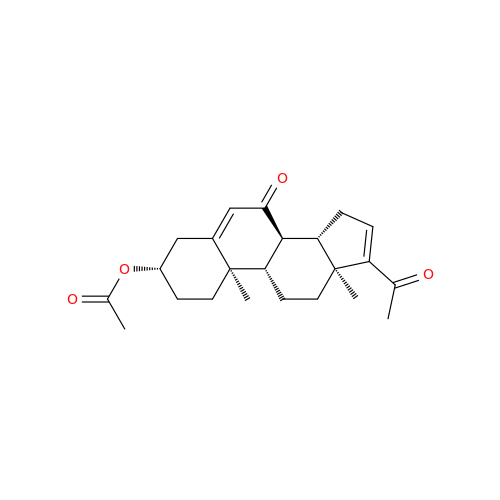
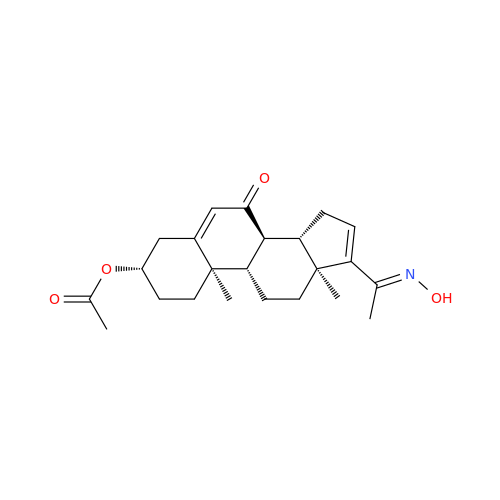
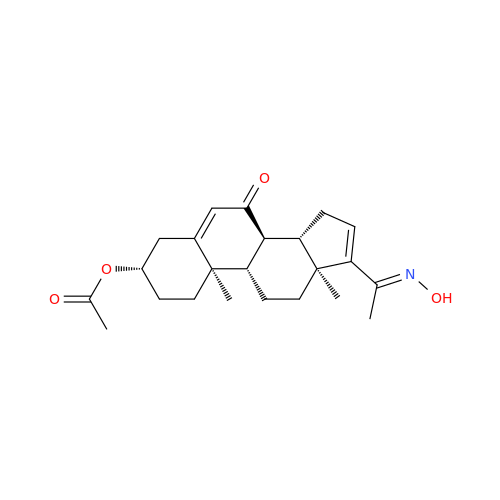
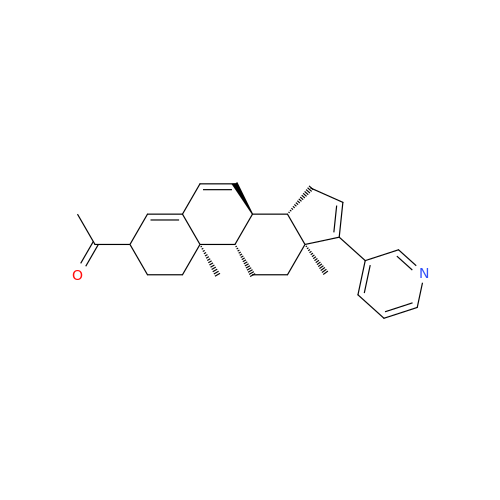
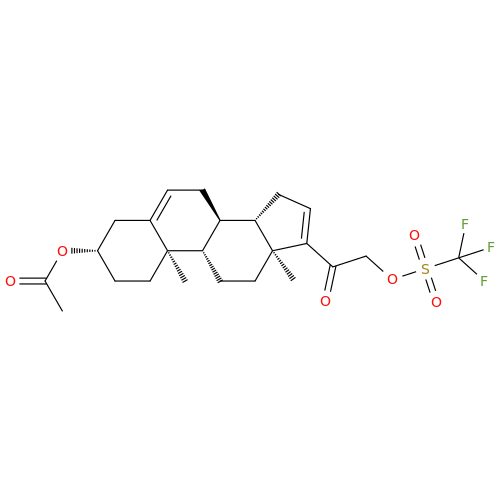
Chemical Name: Abiraterone Acetate Reduced Impurity
Synonym: 2,3,4,5,6,7,8,9,10,11,12,13,14,15-Tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, (3S,8R,9S,10S,13S,14S)-10,13-dimethyl-17-(pyridin-3-yl)| Enter Batch Number | |||