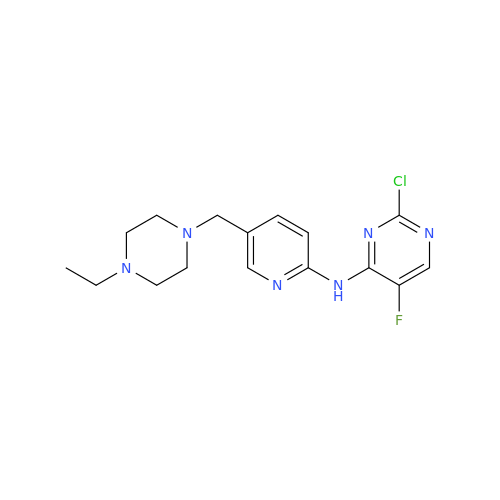
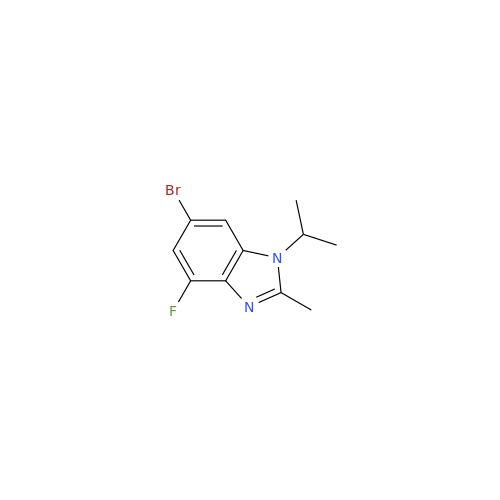
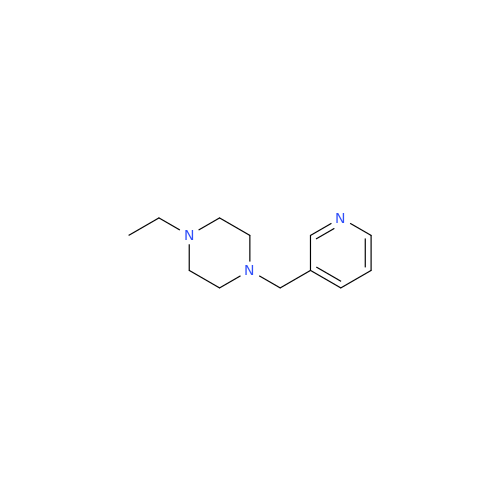
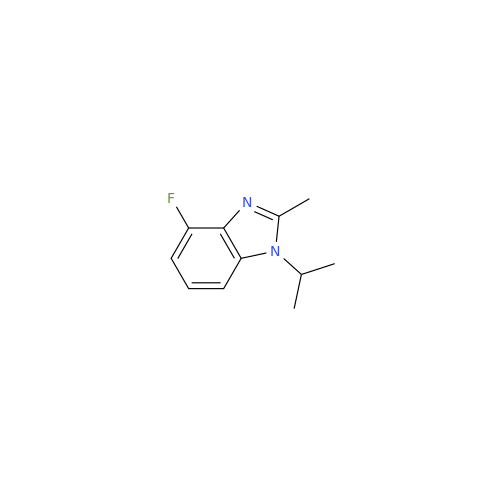
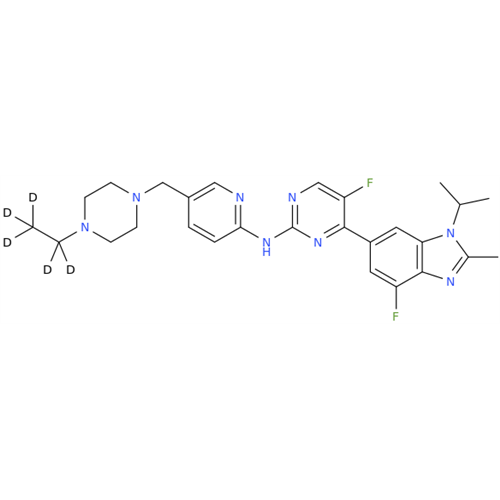
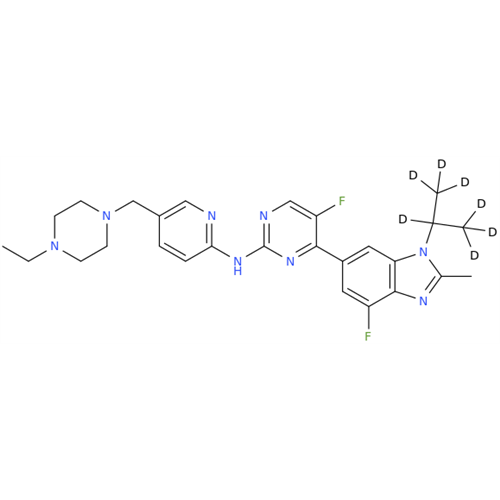
Product Information
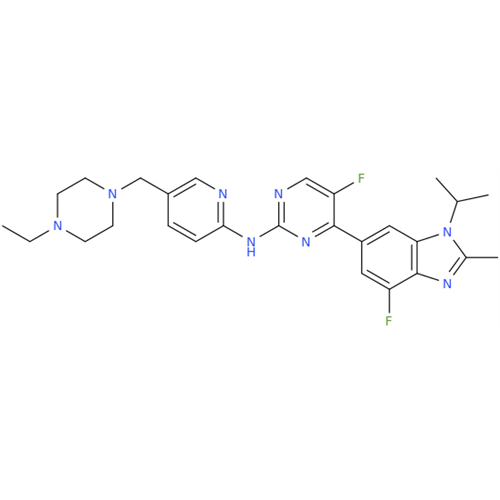
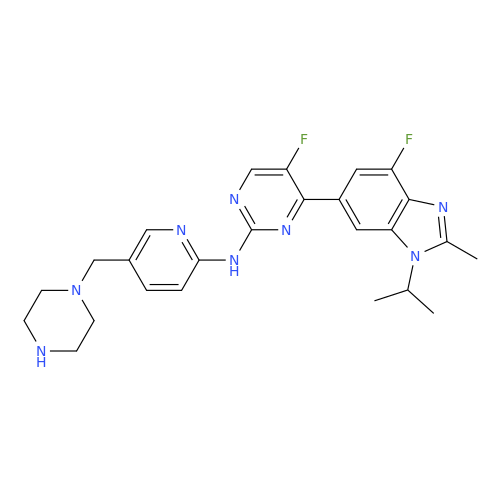
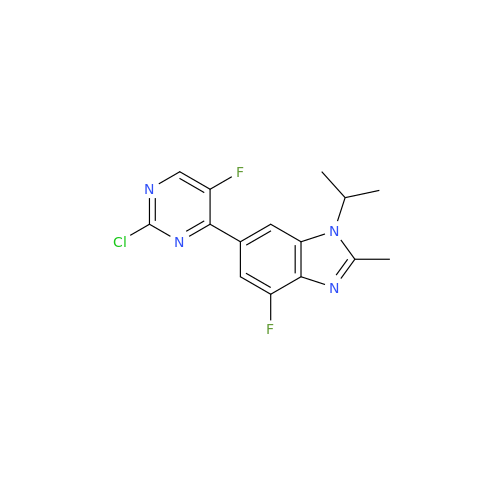
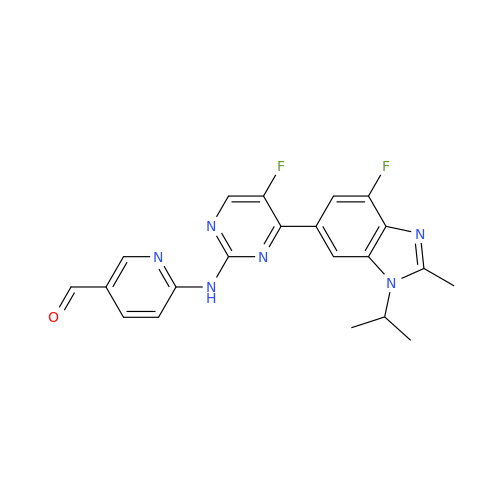
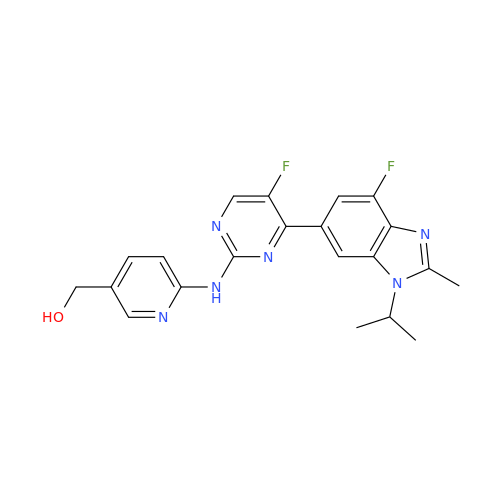
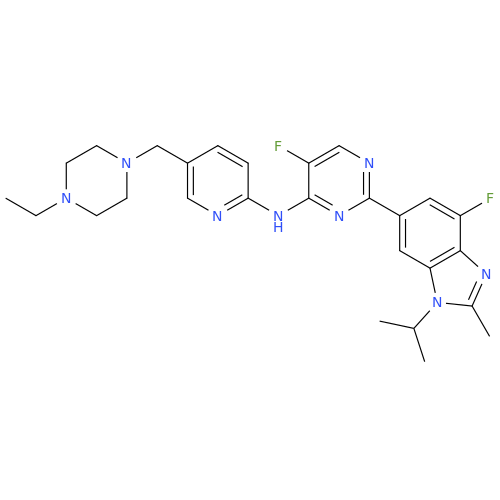
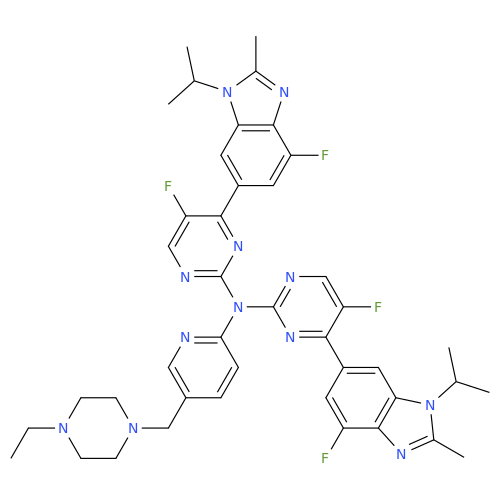
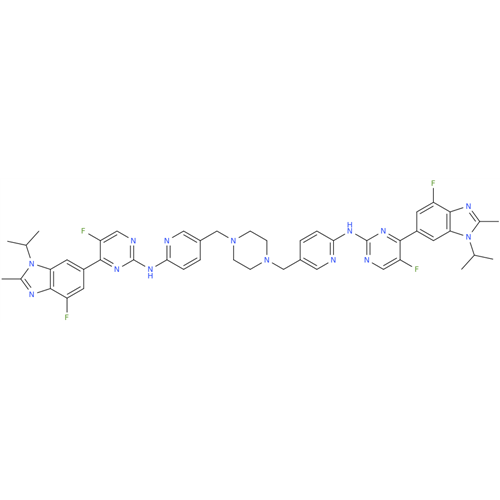
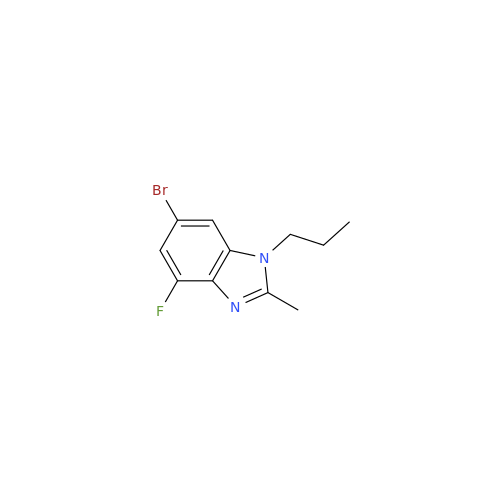
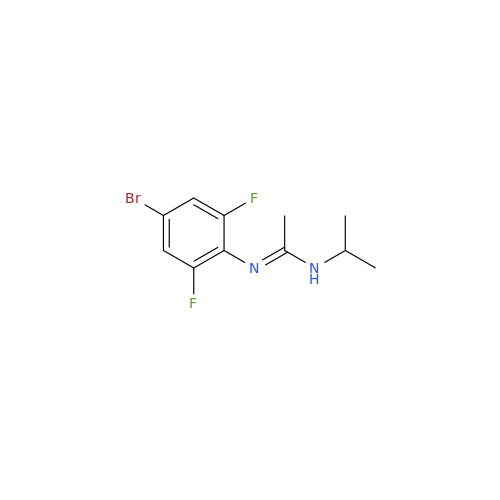
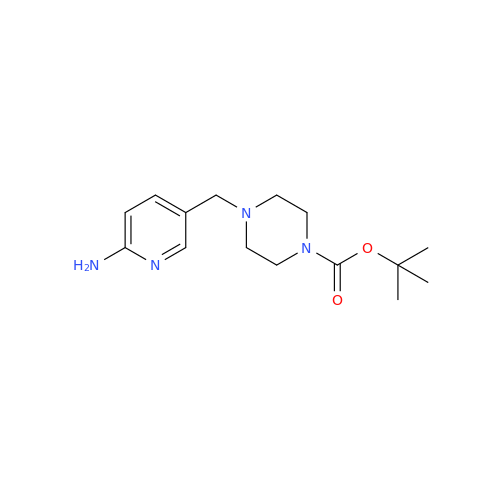
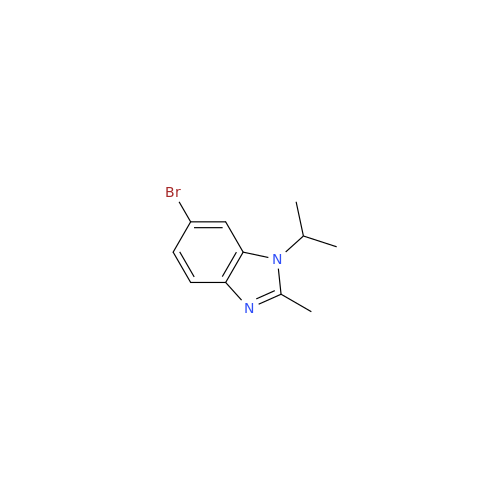
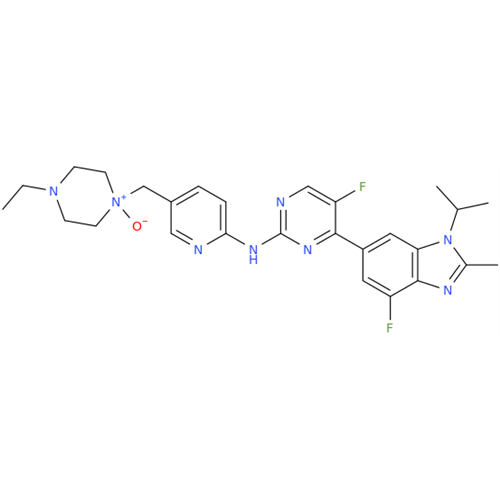
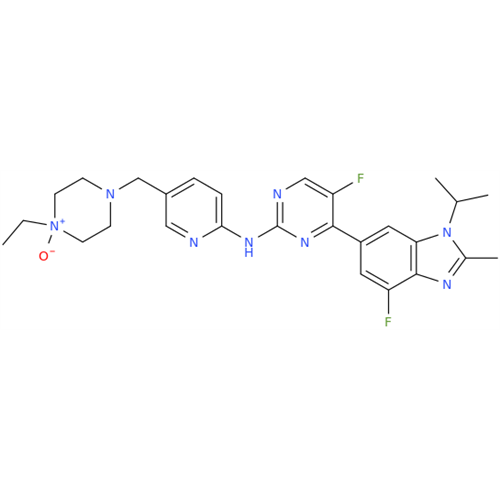
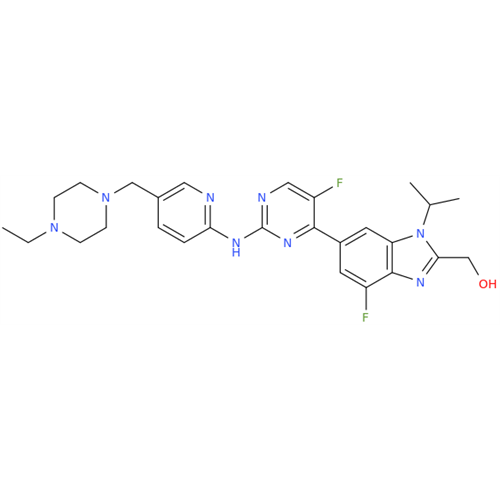
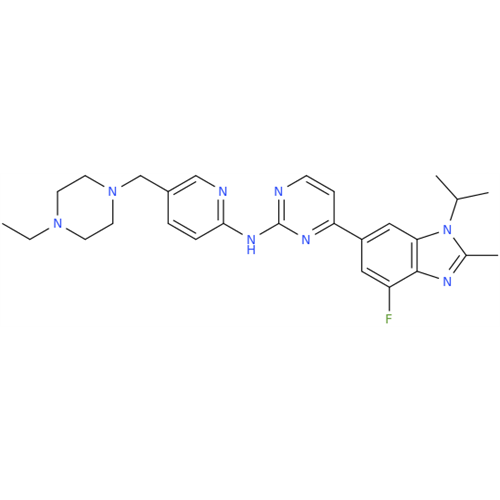
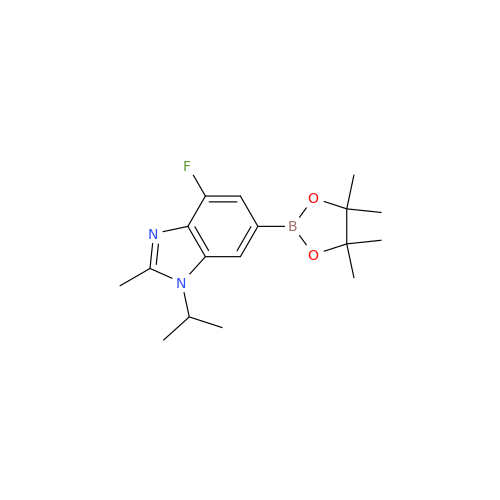
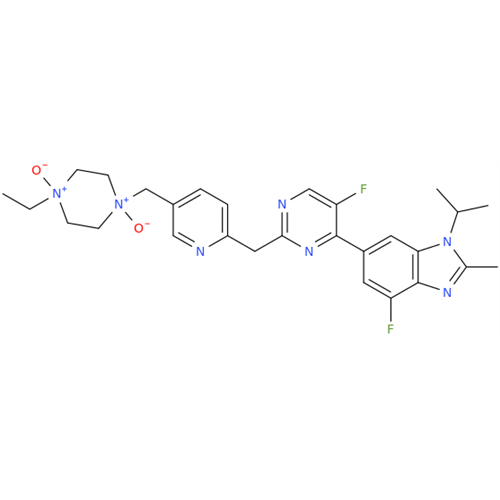
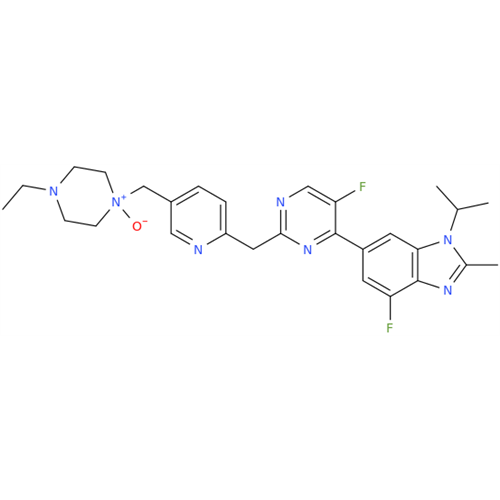
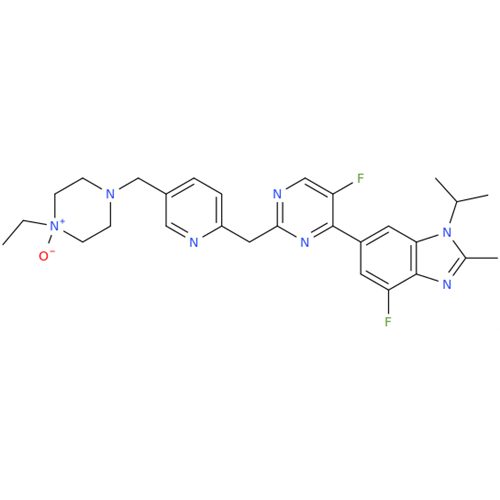
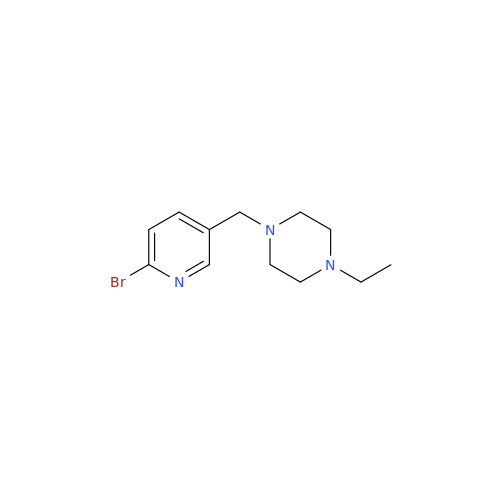
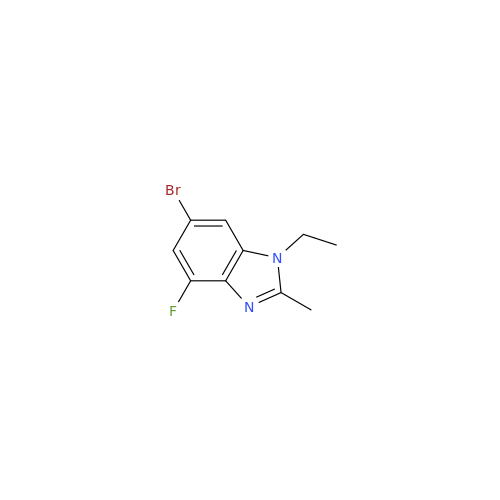
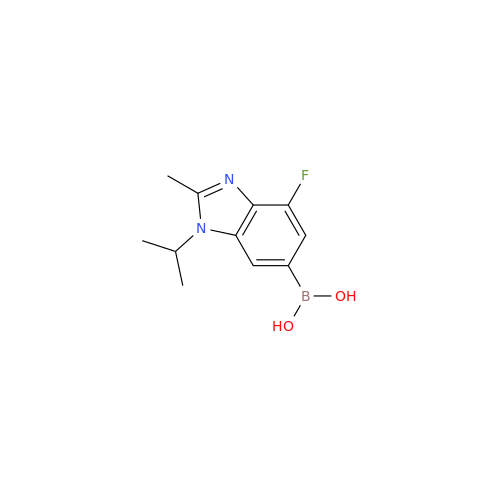
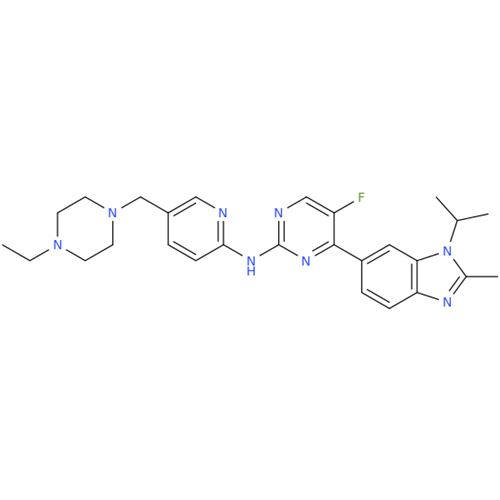
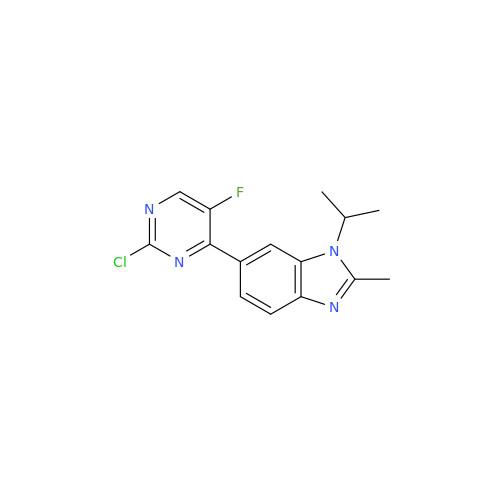
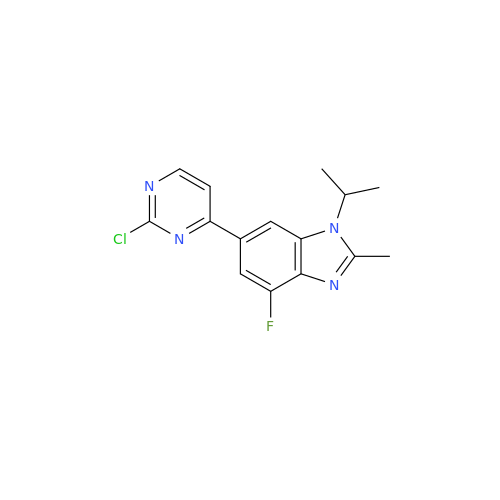
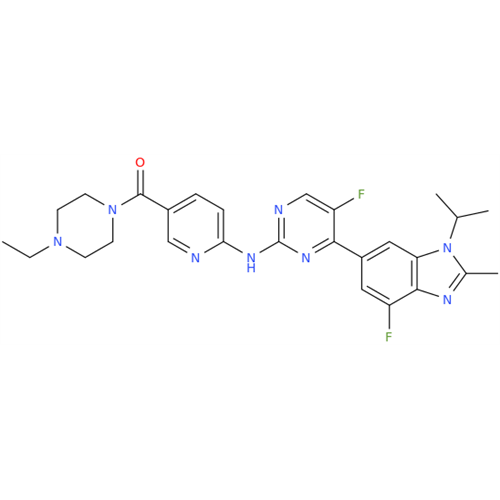
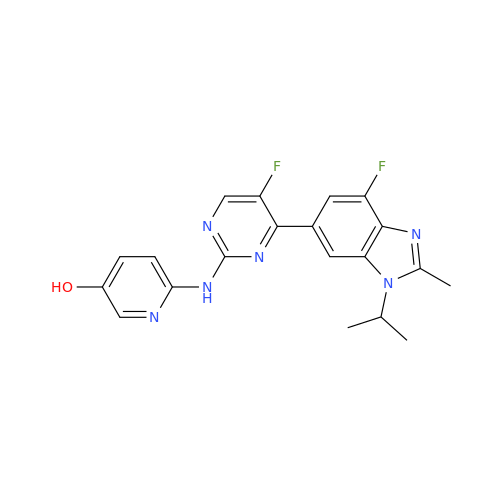
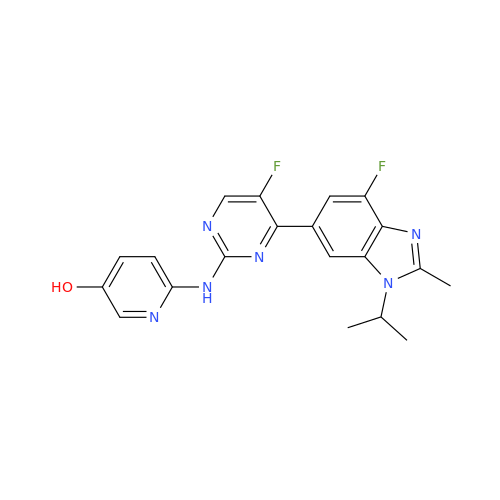
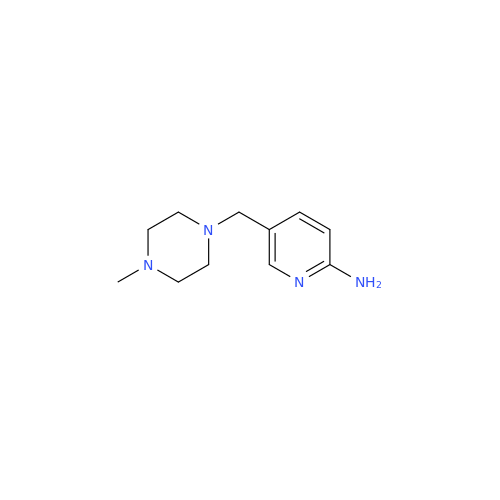
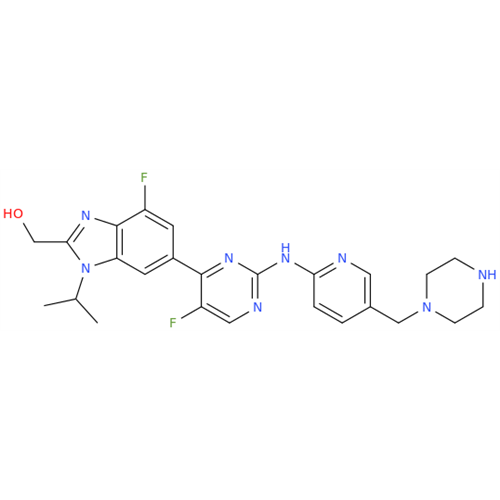
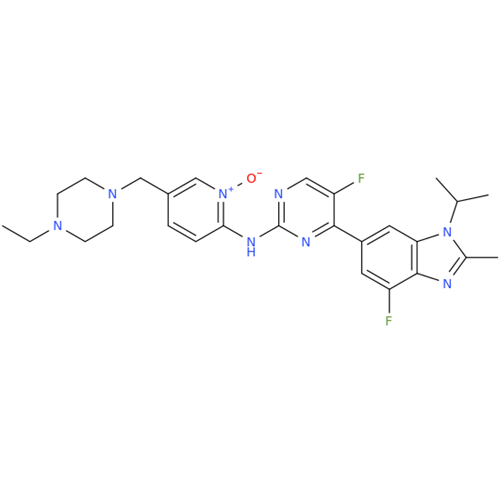
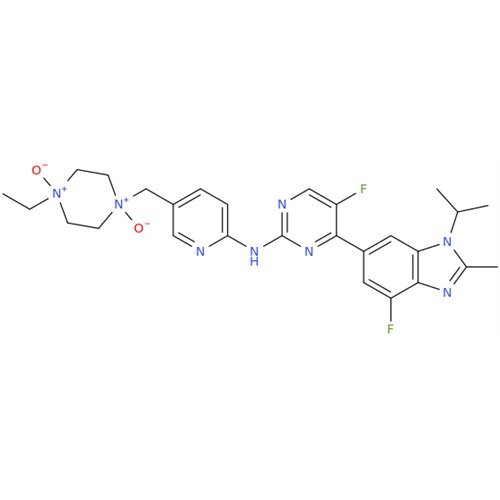
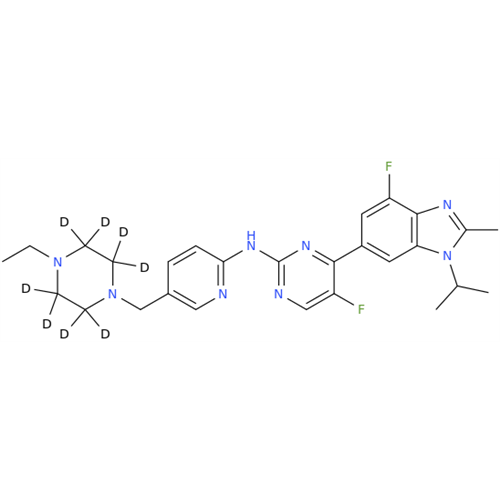
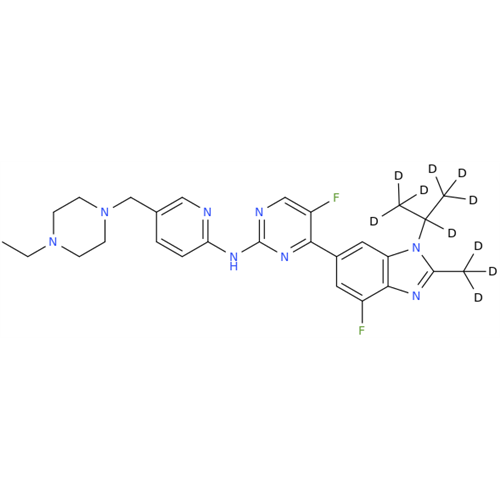
Abemaciclib Impurity 34
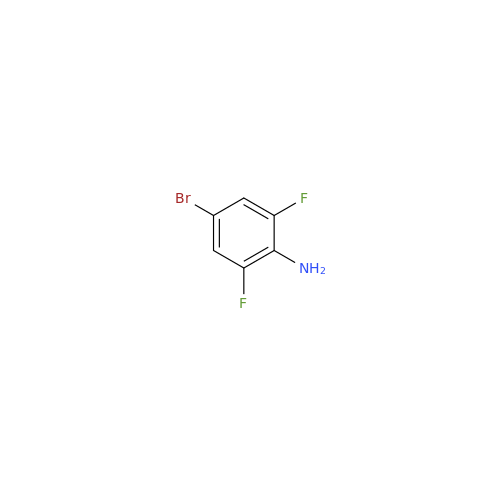
|
Chemical Name: Abemaciclib Impurity 34
Synonym: Benzenamine, 4-bromo-2,6-difluoro; 4-Bromo-2,6-difluorobenzenamine; (4-Bromo-2,6-difluorophenyl)amine; 2,6-Difluoro-4-bromoaniline| Enter Batch Number | |||